Abstract
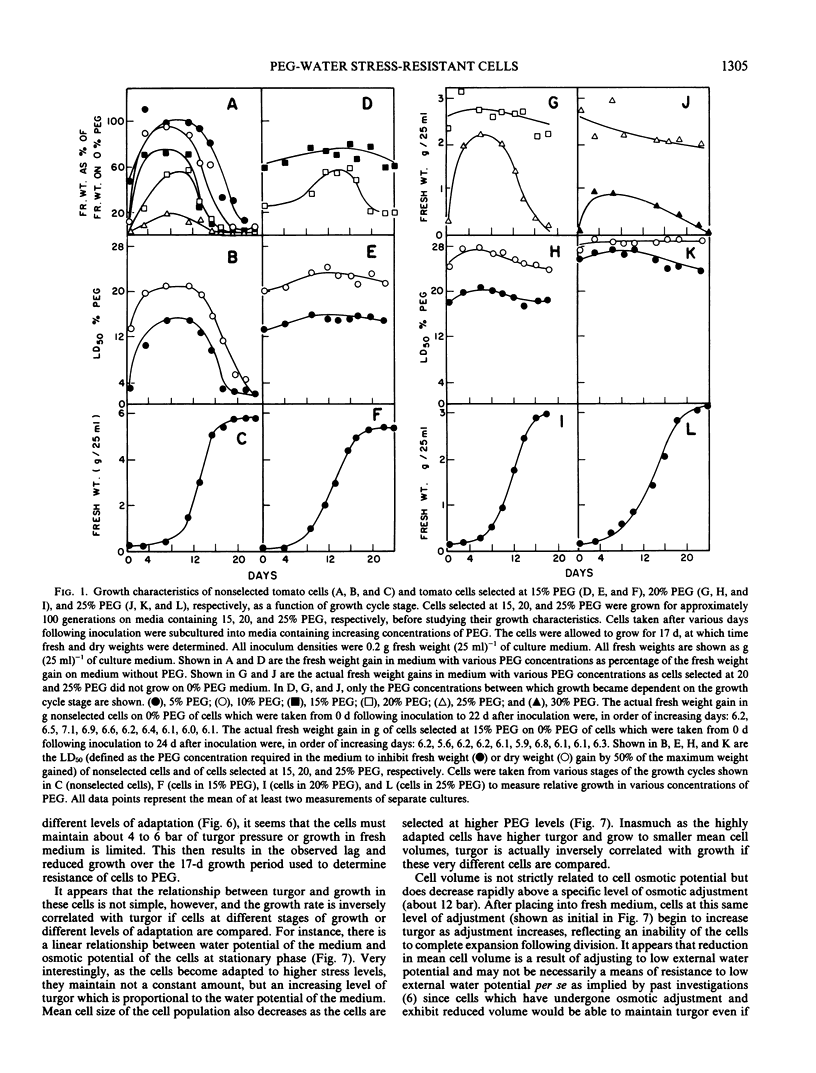
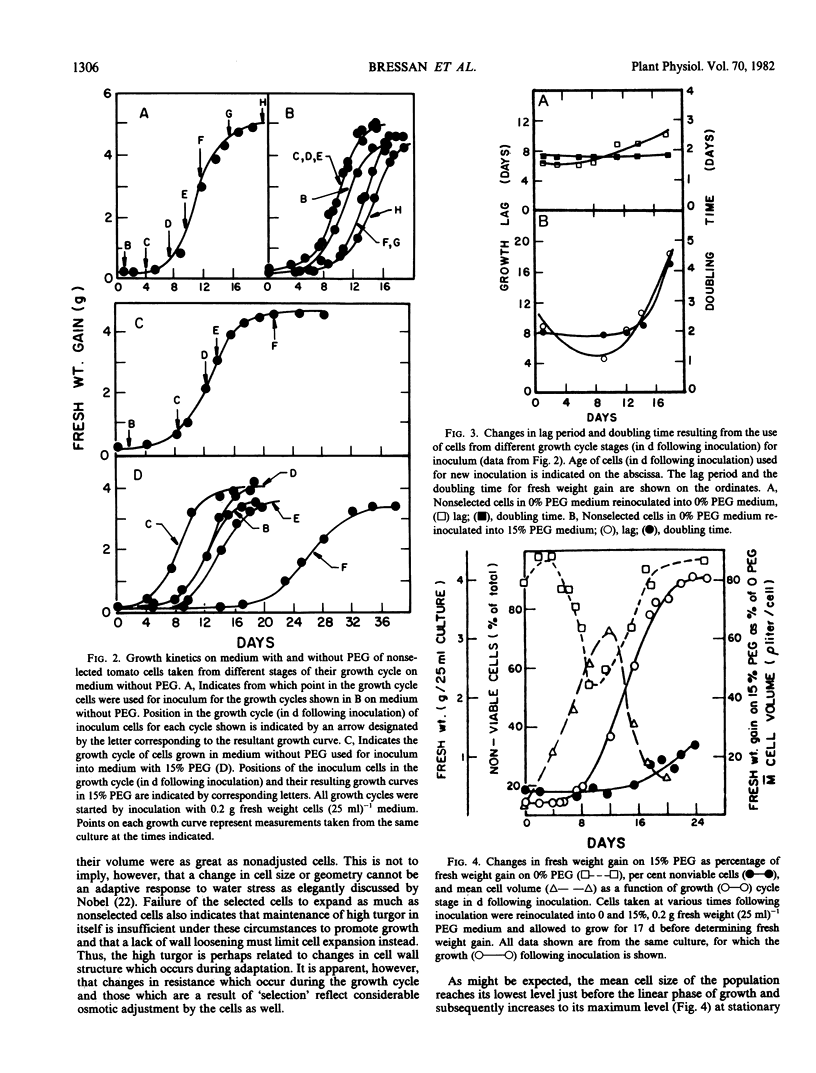
Cultured cells of tomato, Lycopersicon esculentum Mill. cv VFNT-cherry, have been selected for resistance to water stress (low water potential) imposed by the addition of polyethylene glycol to the culture medium. The ability of nonselected cells to grow in media with low water potentials changes dramatically with the age of the cells (with respect to days following inoculation) whereas there is little effect of the age of selected cells on growth over the same media water potentials. The increased resistance of selected cells has limited stability in the absence of stress, indicating that resistance is established by a slow reversible adaptive process.
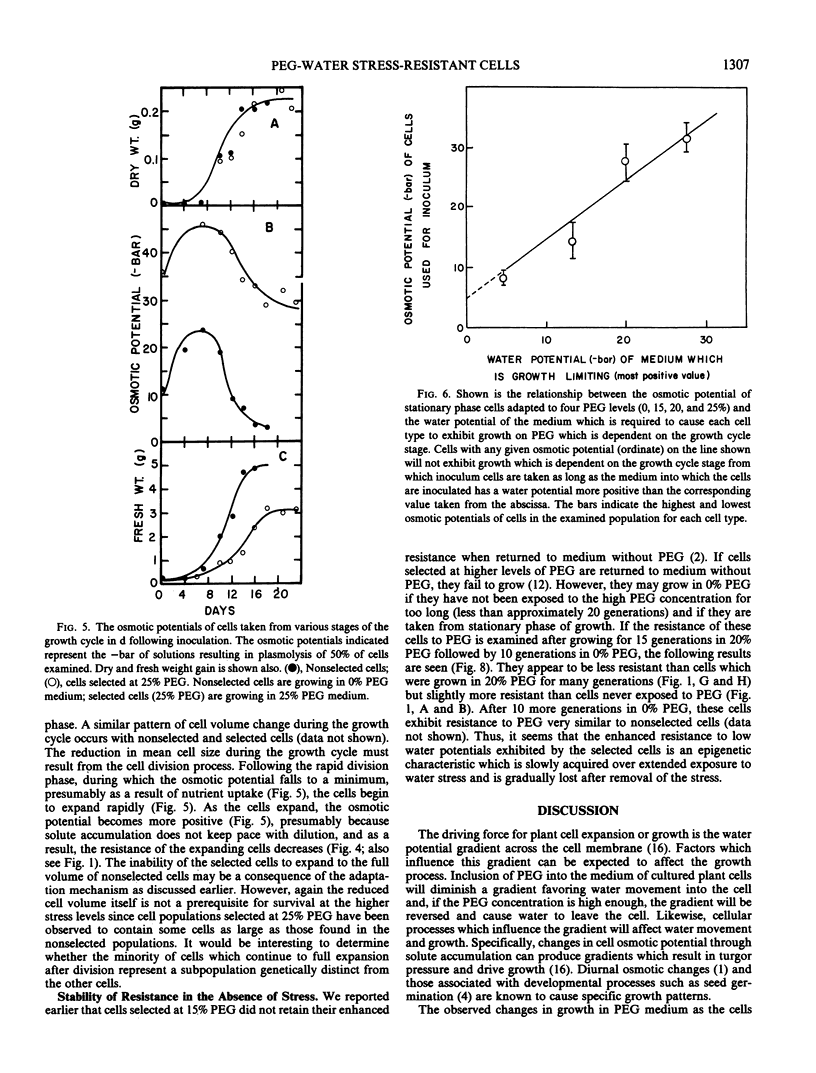
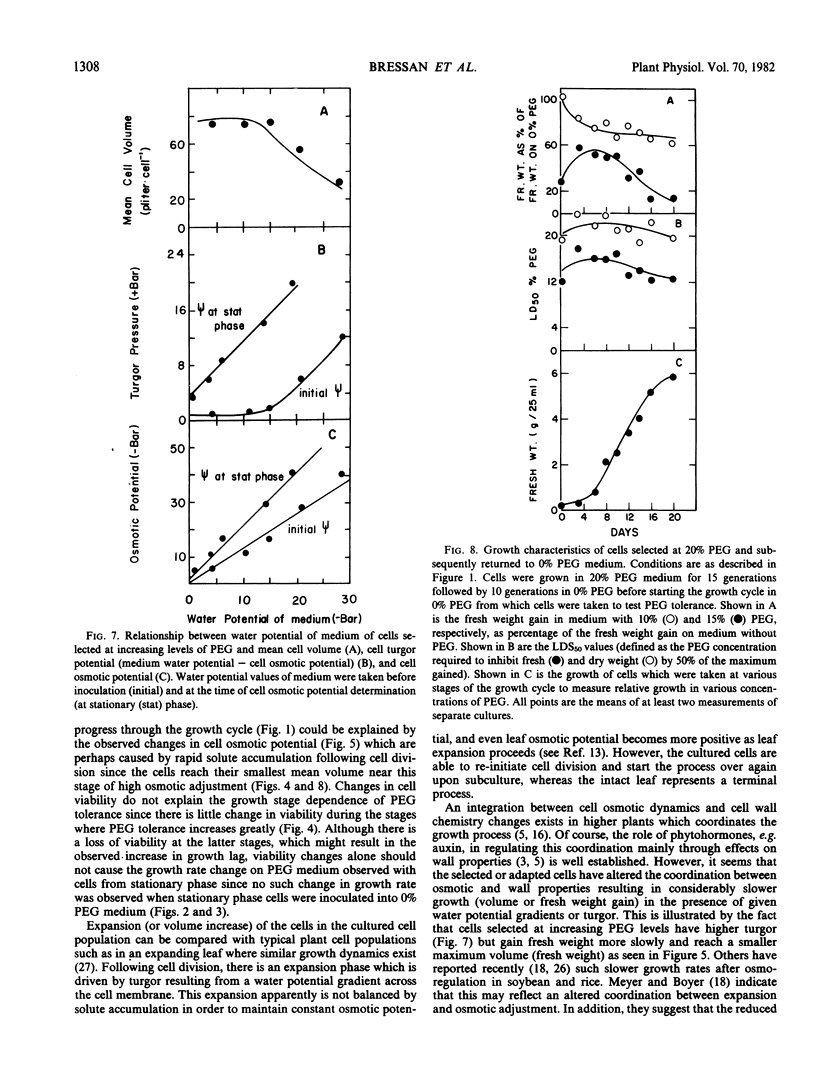
Increased resistance (growth) in the presence of water stress appears to result from considerable osmotic adjustment by the cells. Growth cycle-dependent changes in resistance of nonselected cells are correlated with osmotic potential changes which are associated with the normal cell growth pattern in culture. Lowered osmotic potential is maintained by selected cells throughout the entire growth cycle and may explain the growth cycle independence of growth of selected cells on polyethylene glycol-containing media. Osmotic adjustment of resistant cells at stationary phase can be as much as 40 bar. Turgor is maintained by resistant cells (as high as 21 bar) in media with low water potentials at least partly at the expense of cell expansion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo E., Fereres E., Hsiao T. C., Henderson D. W. Diurnal growth trends, water potential, and osmotic adjustment of maize and sorghum leaves in the field. Plant Physiol. 1979 Sep;64(3):476–480. doi: 10.1104/pp.64.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. The control of cell enlargement. Symp Soc Exp Biol. 1977;31:101–115. [PubMed] [Google Scholar]
- Davies H. M., Miflin B. J. Regulatory isoenzymes of aspartate kinase and the control of lysine and threonine biosynthesis in carrot cell suspension culture. Plant Physiol. 1978 Oct;62(4):536–541. doi: 10.1104/pp.62.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E. Multisample enzyme extraction from cultured plant cell suspensions. Plant Physiol. 1971 Jan;47(1):38–42. doi: 10.1104/pp.47.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Handa A. K., Bressan R. A., Handa S., Hasegawa P. M. Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol. 1982 Feb;69(2):514–521. doi: 10.1104/pp.69.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul B., Staba E. J. Ammi visnaga (L) Lam. Tissue cultures. Multi-liter suspension growth and examination for furanochromones. Planta Med. 1967 May;15(2):145–156. doi: 10.1055/s-0028-1099967. [DOI] [PubMed] [Google Scholar]
- Larue T. A., Gamborg O. L. Ethylene Production by Plant Cell Cultures: Variations in Production during Growing Cycle and in Different Plant Species. Plant Physiol. 1971 Oct;48(4):394–398. doi: 10.1104/pp.48.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckner M., Nover L. Expression of secondary metabolism. An aspect of cell specialization of microorganisms, higher plants, and animals. Mol Biol Biochem Biophys. 1977;23:3–102. [PubMed] [Google Scholar]
- Murphy T. M., Imbrie C. W. Induction and Characterization of Chlorate-resistant Strains of Rosa damascena Cultured Cells. Plant Physiol. 1981 May;67(5):910–916. doi: 10.1104/pp.67.5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargool P. D. Some aspects of the regulation of arginine biosynthesis in soybean cell cultures. Plant Physiol. 1973 Jul;52(1):68–71. doi: 10.1104/pp.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Jayaswal R. K., Johri M. M. Cell-density-dependent Changes in the Metabolism of Chloronema Cell Cultures: I. Relationship between Cell Density and Enzymic Activities. Plant Physiol. 1979 Jul;64(1):154–158. doi: 10.1104/pp.64.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]


