Abstract
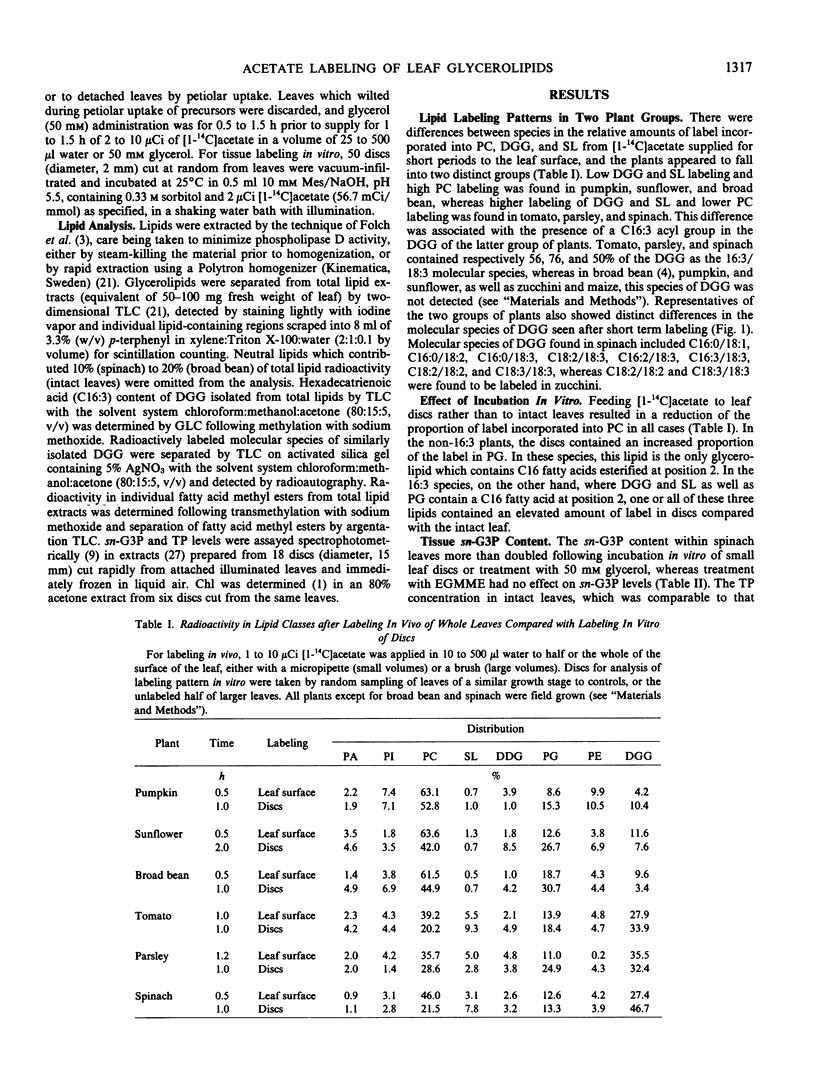
During short term labeling of expanding leaves of seven plant species with [1-14C]acetate, 35 to 64% of the label incorporated into lipids was found in phosphatidylcholine and 5 to 24% in phosphatidylglycerol. In pumpkin, sunflower, broad bean, and maize, only 4 to 12% of the label was found in diacylgalactosylglycerol, but in tomato, parsley, and spinach, the proportion was 17 to 31%. The latter group was further distinguished by having diacylgalactosylglycerol containing C16:3.
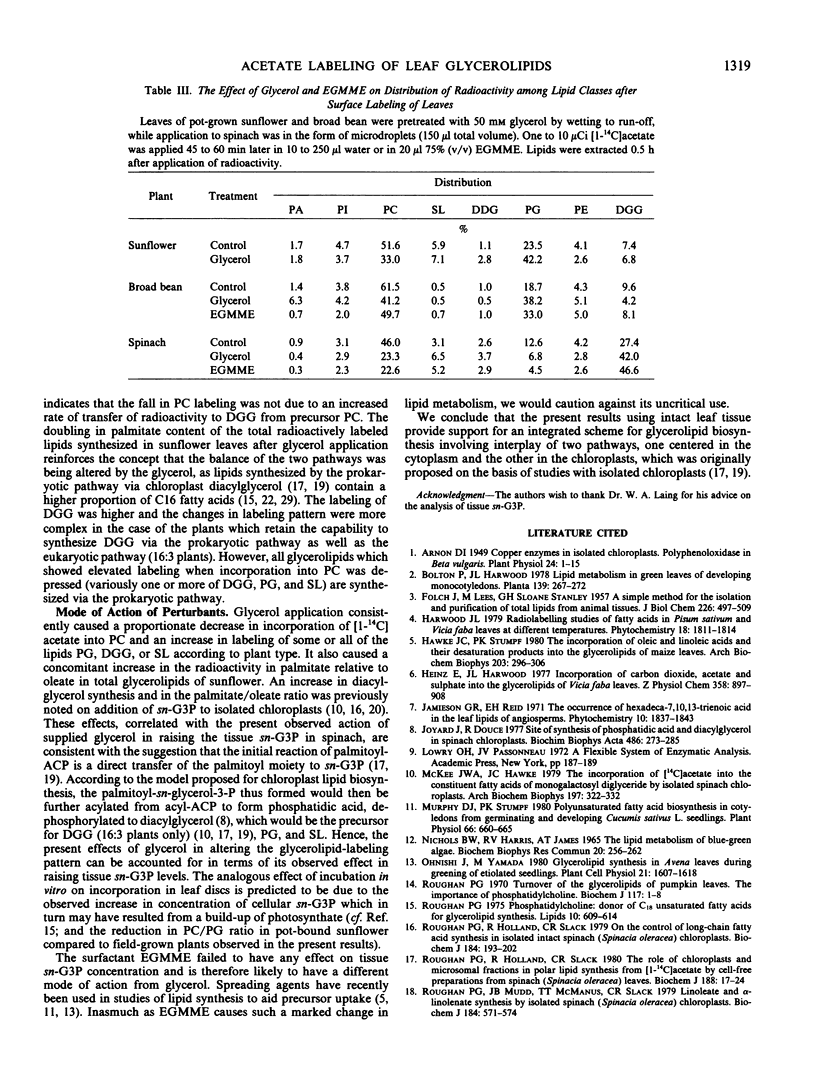
The proportions of total incorporated [1-14C]acetate entering the lipids could be manipulated in a predictable manner. Phosphatidylcholine labeling was depressed by treating intact leaves with glycerol or ethylene glycol monomethyl ether or by incubating leaf discs in vitro. An associated increase in phosphatidylglycerol labeling occurred within the first group of plants, whereas an increase in labeling of either diacylgalactosylglycerol, phosphatidylglycerol, or sulfolipid occurred within the second group. Treating intact leaves with glycerol or incubating leaf discs in vitro was shown to elevate cellular concentrations of sn-glycerol 3-phosphate.
These results have been interpreted in terms of the two-pathway hypothesis for glycerolipid biosynthesis, in which it is proposed that phosphatidylcholine is synthesized via a different pathway (eukaryotic) to that for synthesis of phosphatidylglycerol (prokaryotic). Both pathways may contribute toward the synthesis of diacylgalactosylglycerol, with the contribution of each being assessed from the proportion of hexadecatrienoic acid found in the particular plant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hawke J. C., Stumpf P. K. The incorporation of oleic and linoleic acids and their desaturation products into the glycerolipids of maize leaves. Arch Biochem Biophys. 1980 Aug;203(1):296–306. doi: 10.1016/0003-9861(80)90180-0. [DOI] [PubMed] [Google Scholar]
- Heinz E., Harwood J. L. Incorporation of carbon dioxide, acetate and sulphate into the glycerolipids of Vicia faba leaves. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):897–908. doi: 10.1515/bchm2.1977.358.2.897. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. Polyunsaturated Fatty Acid Biosynthesis in Cotyledons from Germinating and Developing Cucumis sativus L. Seedlings. Plant Physiol. 1980 Oct;66(4):660–665. doi: 10.1104/pp.66.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Mudd J. B., McManus T. T., Slack C. R. Linoleate and alpha-linolenate synthesis by isolated spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Dec 15;184(3):571–574. doi: 10.1042/bj1840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidyl choline: Donor of 18-carbon unsaturated fatty acids for glycerolipid biosynthesis. Lipids. 1975 Oct;10(10):609–614. doi: 10.1007/BF02532725. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Joyard J., Douce R. Labelling in vivo and in vitro of molecular species of lipids from chloroplast envelopes and thylakoids. Eur J Biochem. 1980;108(1):177–185. doi: 10.1111/j.1432-1033.1980.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Siebertz M., Heinz E. Galactosylation of different monogalactosyldiacylglycerols by cell-free preparations from pea leaves. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):27–34. doi: 10.1515/bchm2.1977.358.1.27. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Wharfe J., Harwood J. L. Fatty acid biosynthesis in the leaves of barley, wheat and pea. Biochem J. 1978 Jul 15;174(1):163–169. doi: 10.1042/bj1740163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Leung S. P. Galactolipid Synthesis in Vicia faba Leaves: II. Formation and Desaturation of Long Chain Fatty Acids in Phosphatidylcholine, Phosphatidylglycerol, and the Galactolipids. Plant Physiol. 1976 Feb;57(2):179–184. doi: 10.1104/pp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]



