Abstract
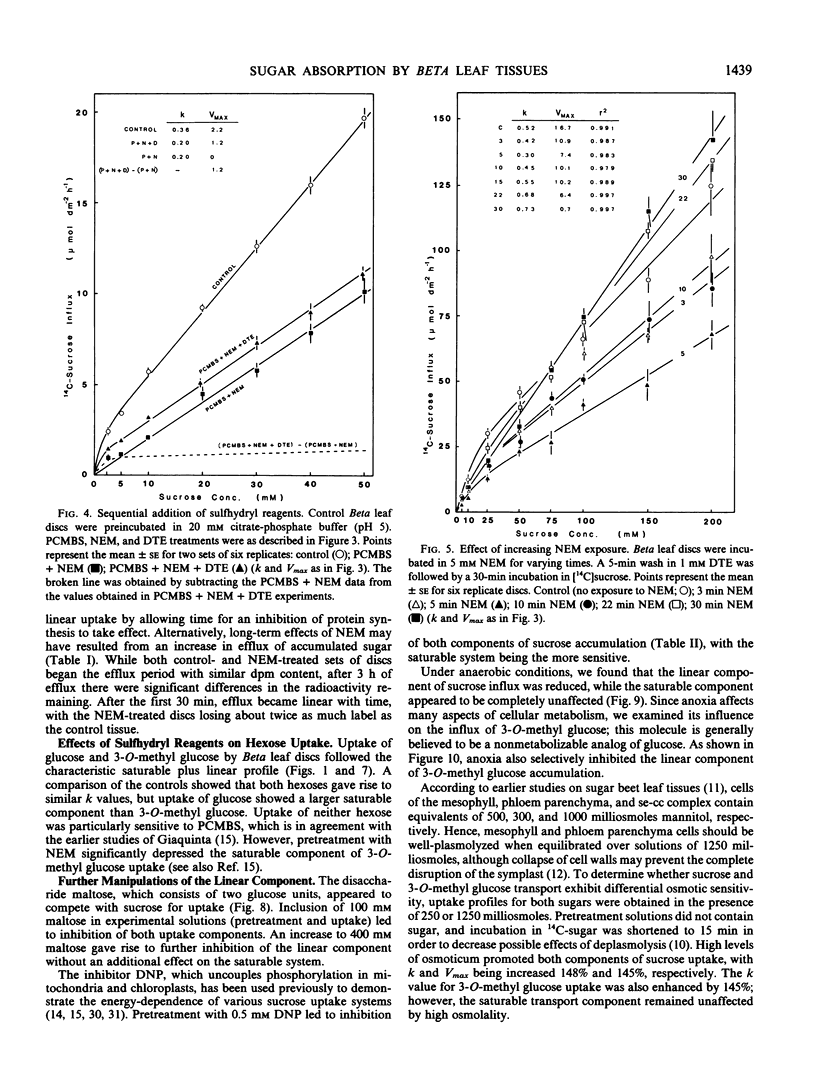
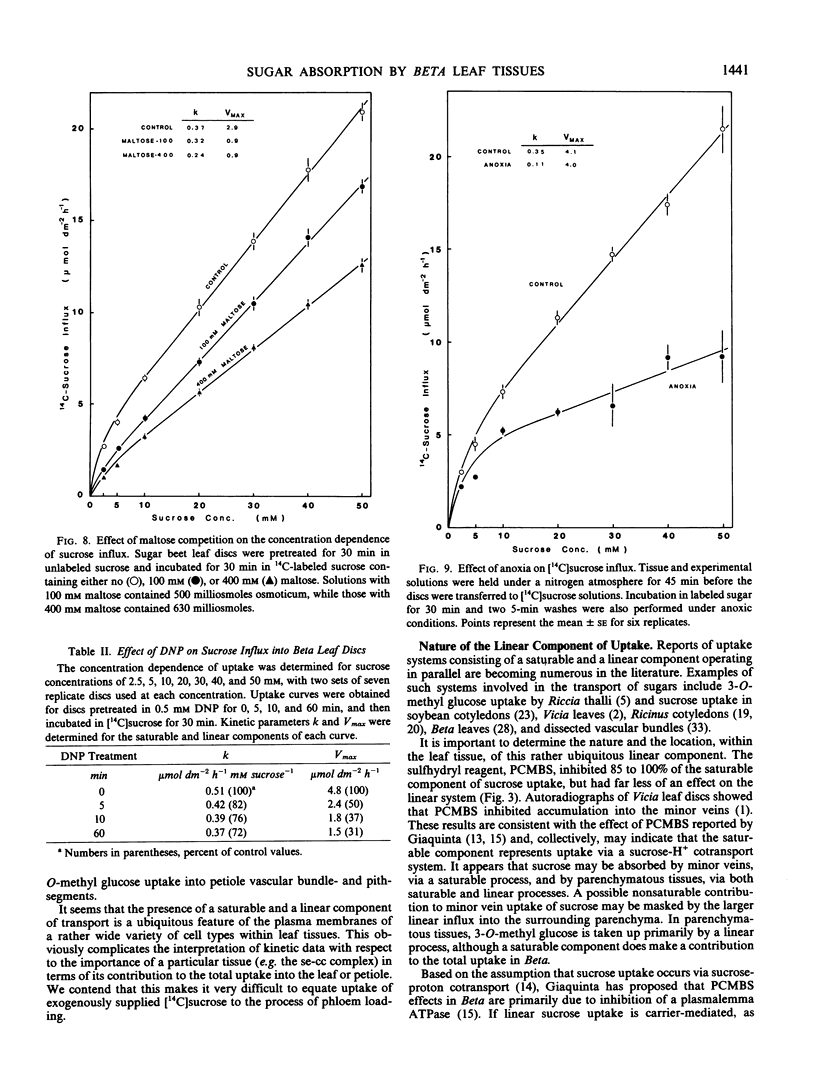
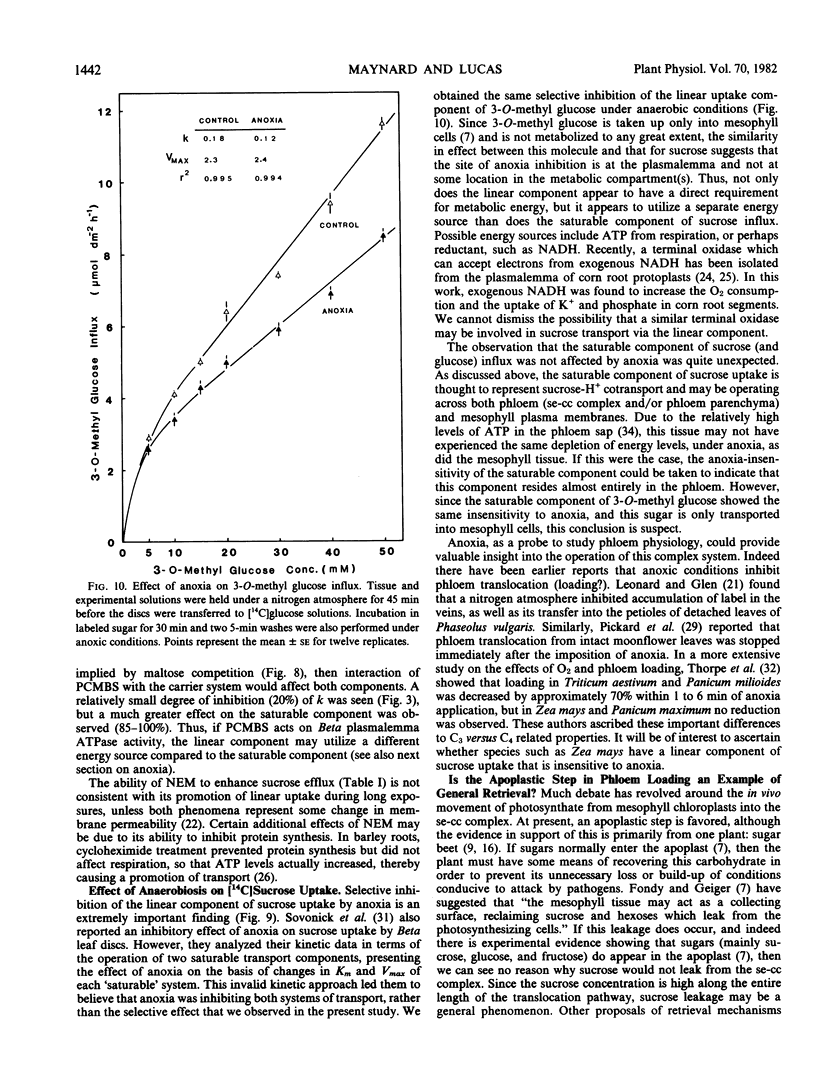
Concentration curves for sugar and amino acid uptake by Beta vulgaris L. leaf tissues contained both a saturable and a linear component. Similarly shaped curves were obtained for influx of sucrose, glucose, and 3-O-methyl glucose by leaf discs, whole petiole slices, petiole segments containing pith tissue only, and petiole segments containing vascular bundles, although the tissues took up the various sugars via different proportions of saturable versus linear uptake. Two millimolar p-chloromercuribenzenesulfonic acid selectively inhibited the saturable component of sucrose uptake, but had almost no effect on the linear component. Uptake of glucose and 3-O-methyl glucose remained unaffected by p-chloromercuribenzenesulfonic acid treatment. Anoxia was found to inhibit the linear component of both sucrose and 3-O-methyl glucose influx, while the saturable component remained unaffected. The linear component of sucrose uptake was also competitively inhibited by maltose, as well as being selectively promoted by certain exposures to 5 millimolar N-ethylmaleimide, 2 micrograms per milliliter cycloheximide, and high levels of mannitol acting as osmoticum. These results support the proposal that the linear component is due to a process more complex than simple, or exchange, diffusion. It would also appear that the linear transport component utilizes a separate energy source than does the saturable component of sucrose influx.
Evidence for phloem loading from the apoplast was re-examined with respect to the present findings. Saturable sucrose uptake by minor vein tissues may represent retrieval of solute from the free space, which could explain the `apoplastic loading' phenomenon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delrot S., Bonnemain J. L. Involvement of Protons as a Substrate for the Sucrose Carrier during Phloem Loading in Vicia faba Leaves. Plant Physiol. 1981 Mar;67(3):560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S. Proton Fluxes Associated with Sugar Uptake in Vicia faba Leaf Tissues. Plant Physiol. 1981 Sep;68(3):706–711. doi: 10.1104/pp.68.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Sugar Selectivity and Other Characteristics of Phloem Loading in Beta vulgaris L. Plant Physiol. 1977 May;59(5):953–960. doi: 10.1104/pp.59.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno M., Carrasco L., Vazquez D. Initiation of the polypeptide chain by reticulocyte cell-free systems. Survey of different inhibitors of translation. Eur J Biochem. 1976 Sep 15;68(2):355–364. doi: 10.1111/j.1432-1033.1976.tb10822.x. [DOI] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979 Apr;63(4):744–748. doi: 10.1104/pp.63.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder J., Cronshaw J. A biochemical and cytochemical study of adenosine triphosphatase activity in the phloem of Nicotiana tabacum. J Cell Biol. 1974 Jan;60(1):221–235. doi: 10.1083/jcb.60.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Moreland D. E. Translocation: EFFLUX OF SUGARS ACROSS THE PLASMALEMMA OF MESOPHYLL PROTOPLASTS. Plant Physiol. 1980 Mar;65(3):560–562. doi: 10.1104/pp.65.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard O. A., Glenn R. K. Translocation of assimilates and phosphate in detached bean leaves. Plant Physiol. 1968 Sep;43(9):1380–1388. doi: 10.1104/pp.43.9.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Sucrose uptake by developing soybean cotyledons. Plant Physiol. 1981 Sep;68(3):693–698. doi: 10.1104/pp.68.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Isolation of NADH Oxidation System from the Plasmalemma of Corn Root Protoplasts. Plant Physiol. 1982 Jul;70(1):326–328. doi: 10.1104/pp.70.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Responses of corn root protoplasts to exogenous reduced nicotinamide adenine dinucleotide: Oxygen consumption, ion uptake, and membrane potential. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3773–3776. doi: 10.1073/pnas.79.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Läuchli A., Ball E., Pitman M. G. Cycloheximide: a specific inhibitor of protein synthesis and intercellular ion transport in plant roots. Experientia. 1974 May 15;30(5):470–471. doi: 10.1007/BF01926298. [DOI] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. A Reanalysis of the Two-Component Phloem Loading System in Beta vulgaris. Plant Physiol. 1982 Mar;69(3):734–739. doi: 10.1104/pp.69.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Schrader L. E., Jung D. M. Energy-dependent Loading of Amino Acids and Sucrose into the Phloem of Soybean. Plant Physiol. 1979 Oct;64(4):546–550. doi: 10.1104/pp.64.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


