Abstract
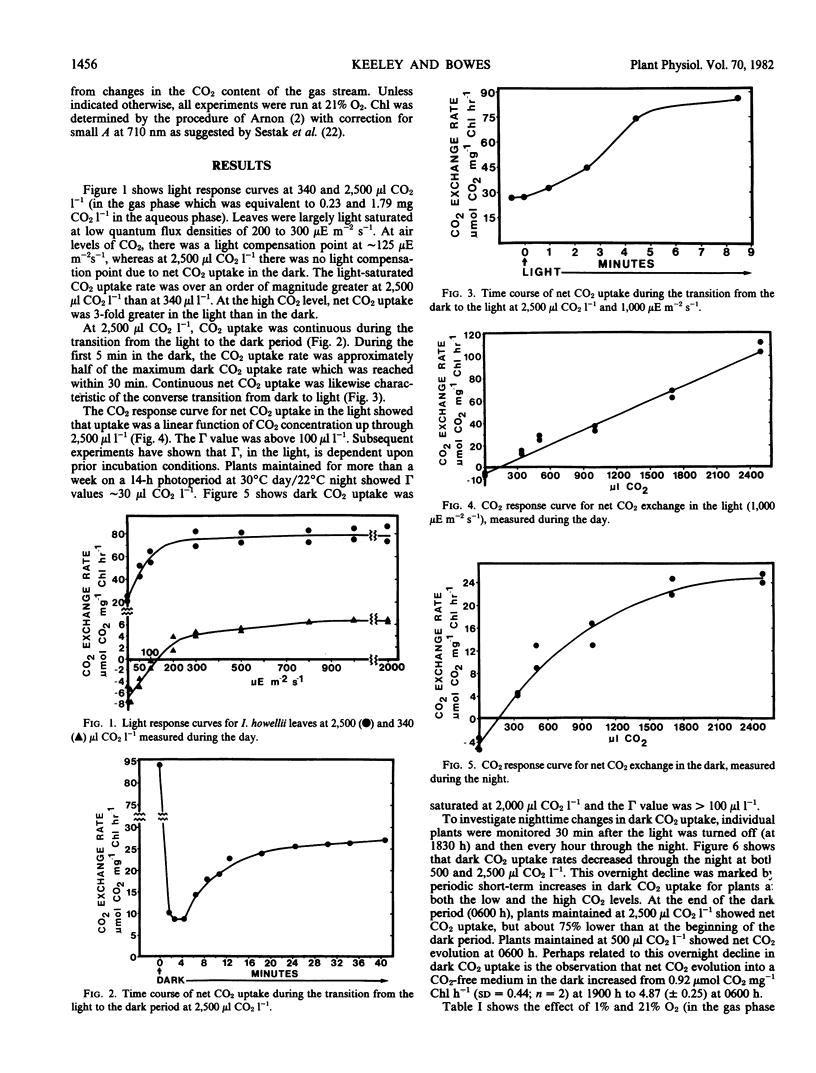
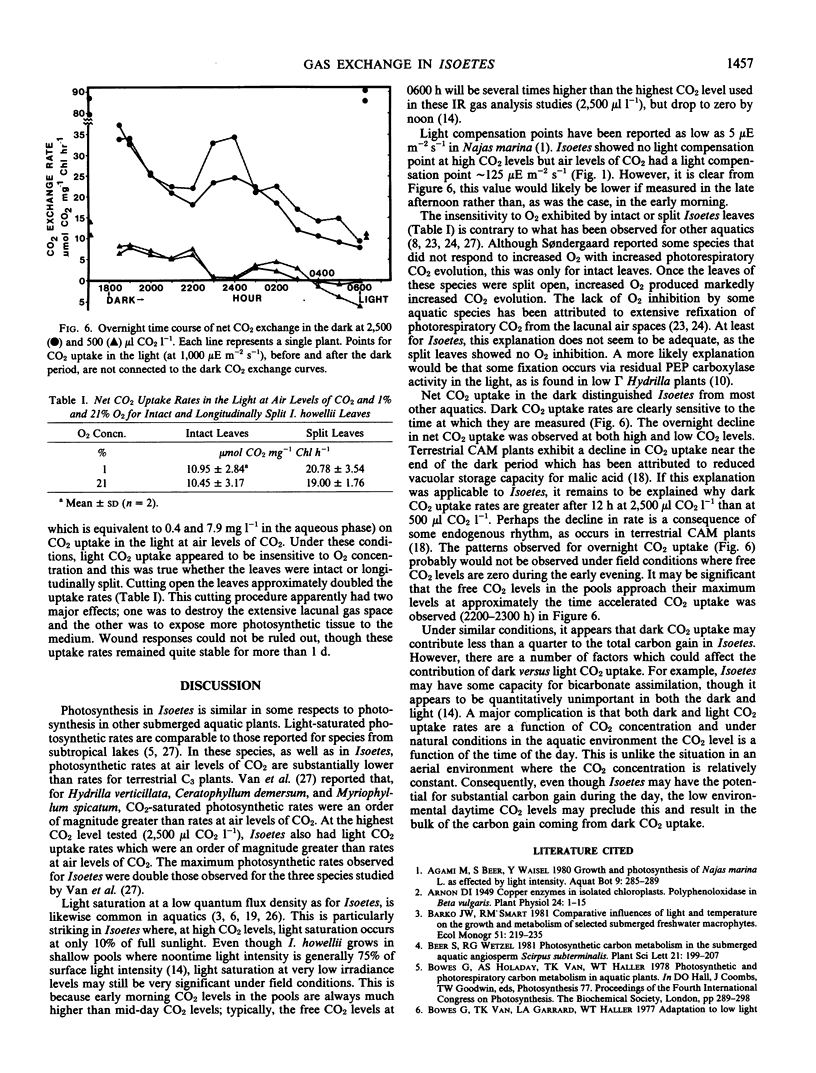
The submerged aquatic plant Isoetes howellii Engelmann possesses Crassulacean acid metabolism (CAM) comparable to that known from terrestrial CAM plants. Infrared gas analysis of submerged leaves showed Isoetes was capable of net CO2 uptake in both light and dark. CO2 uptake rates were a function of CO2 levels in the medium. At 2,500 microliters CO2 per liter (gas phase, equivalent to 1.79 milligrams per liter aqueous phase), Isoetes leaves showed continuous uptake in both the light and dark. At this CO2 level, photosynthetic rates were light saturated at about 10% full sunlight and were about 3-fold greater than dark CO2 uptake rates. In the dark, CO2 uptake rates were also a function of length of time in the night period. Measurements of dark CO2 uptake showed that, at both 2,500 and 500 microliters CO2 per liter, rates declined during the night period. At the higher CO2 level, dark CO2 uptake rates at 0600 h were 75% less than at 1800 h. At 500 microliters CO2 per liter, net CO2 uptake in the dark at 1800 h was replaced by net CO2 evolution in the dark at 0600 h. At both CO2 levels, the overnight decline in net CO2 uptake was marked by periodic bursts of accelerated CO2 uptake. CO2 uptake in the light was similar at 1% and 21% O2, and this held for leaves intact as well as leaves split longitudinally. Estimating the contribution of light versus dark CO2 uptake to the total carbon gain is complicated by the diurnal flux in CO2 availability under field conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W., Sheldon R. B. Submergent macrophytes: growth under winter ice cover. Science. 1976 Nov 19;194(4267):841–842. doi: 10.1126/science.194.4267.841. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Bowes G. Induction of reduced photorespiratory activity in submersed and amphibious aquatic macrophytes. Plant Physiol. 1981 Feb;67(2):335–340. doi: 10.1104/pp.67.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M. CO(2) Fixation in Opuntia Roots. Plant Physiol. 1966 Mar;41(3):500–505. doi: 10.1104/pp.41.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]


