Abstract
It is known that virtually all healthy adult dogs and cats harbor spiral helicobacters in their gastric mucosa. Three species, Helicobacter felis, Helicobacter bizzozeronii, and Helicobacter salomonis have been isolated in vitro from the gastric mucosa of these animals. The aims of this study were to evaluate the efficacy of an isolation method for canine and feline gastric helicobacters that has been developed at the University of Helsinki; to estimate the prevalence and distribution of these taxa in the samples examined; and to assess the efficacy and validity of an extensive set of standardized conventional phenotypic tests, whole-cell protein profiling, and ultrastructural analysis in identifying the different species isolated from canine and feline gastric mucosa. We cultured 95 and 22 gastric mucosal biopsies from dogs and cats, respectively. Twenty-one H. bizzozeronii strains, 8 H. felis strains, 8 H. salomonis strains, 3 mixed cultures, 2 “Flexispira rappini”-like organisms, and 3 as yet uncharacterized strains were isolated from the dogs, and 3 H. felis strains were isolated from the cats. The methods used here yielded Helicobacter isolation rates of 51% from dogs and 13.6% from cats, which exceed those reported previously. The main difficulties were primary isolation, mixed cultures, and identification to the species level. In the species identification, a detailed morphological examination was found to yield important phenotypic characteristics. A large panel of biochemical and tolerance tests did not clearly differentiate the closely related species H. bizzozeronii, H. felis, and H. salomonis. Highly standardized whole-cell protein profiling was shown to be an excellent method for species identification. Improvements in culture conditions for these bacteria are still needed, especially for cats. A genetic identification method not requiring culture is needed for future studies of these very fastidious helicobacters, as the clinical significance and ecology of these species within the gastric mucosa of the domestic carnivores remain largely unknown.
The isolation of Helicobacter pylori, a spiral bacterium from the human gastric mucosa (54) and the subsequent recognition of its prevalence and clinical significance as a cause of gastric ulcers and related diseases in humans (10) led to an increased interest in similar organisms that had been observed in animals (notably domestic pets) over a century ago (49). As a consequence, several novel Helicobacter spp. from the gastric mucosa of various animals, including cheetahs (Helicobacter acinonychis, formerly Helicobacter acinonyx), ferrets (Helicobacter mustelae), monkeys (Helicobacter nemestrinae), and rodents (Helicobacter muridarum) have been described (5, 12, 15, 31). Moreover, three species from cats and dogs, Helicobacter felis, Helicobacter bizzozeronii, and Helicobacter salomonis (19, 26, 46) have been described, while “Flexispira rappini” (also called “Helicobacter rappini”), originally isolated from ovine abortions (28) and subsequently from humans with gastroenteritis (2) and laboratory mice (50), has also been found in canine gastric mucosa (11, 32).
The initial interest in animal helicobacters arose from the need for a suitable animal model for studying H. pylori infection, and subsequently from an ecological perspective (14, 29). However, there have been recent concerns regarding the potential of animals, notably domestic pets, to be a source of zoonotic Helicobacter infection. Cats used for biomedical research have been occasionally found to harbor H. pylori strains (18), while H. felis has been implicated as a potential human pathogen in a few cases (17, 57). In addition, the morphologically distinctive, tightly coiled bacteria (referred to as either “Gastrospirillum hominis” [34] or “Helicobacter heilmannii” [51]) observed in some cases of human gastritis are ultrastructurally indistinguishable from H. bizzozeronii (19) and also from atypical H. felis isolates from which cell surface periplasmic fibrils are absent (11). There is therefore a need to determine the relative prevalence of each species in domestic pets in order to evaluate the possible risk to human health, and also to that of the host animals, in which gastric and related complaints can occur.
Considerable difficulties in the isolation of these organisms from pet animals have been noted. A recent study yielded helicobacter isolation rates of just 11.1%, despite spiral bacteria being observed in 90.7% of the samples under study (11). In addition, there are significant problems in accurately identifying Helicobacter spp. (37), and for prevalence studies to be adequately informative, accurate and preferably simple identification methods should be available. In this regard, both conventional phenotypic tests (44) and whole-cell protein profiling (8) have previously been determined to be effective means for differentiating various Helicobacter spp., while ultrastructural differences between species may also provide important taxonomic data (26, 31).
The purpose of the present study was to evaluate the efficacy of an isolation method for canine and feline gastric helicobacters that has been developed at the University of Helsinki; to assess the efficacy and validity of conventional phenotypic characterization (by an extensive set of standardized tests), whole-cell protein profiling, and ultrastructural analysis in identifying the different species found in canine and feline gastric mucosa; and to estimate the prevalence and distribution of these taxa in the samples examined.
MATERIALS AND METHODS
Animals.
During the period 1990 to 1997, the Faculty of Veterinary Medicine examined 95 canine gastric mucosal biopsies from 37 clinically healthy pet dogs, 23 patients with upper gastrointestinal signs (vomiting, nausea, or abdominal discomfort), 18 euthanized pet dogs (health status unknown), and 17 healthy experimental dogs. Feline gastric biopsies were taken from 22 cats, of which five were clinically healthy, two suffered from upper gastrointestinal problems, and 15 were euthanized pets (health status unknown). The biopsy samples were taken from the corpus (body) area under light anesthesia via endoscope from the live animals and immediately post mortem from the euthanized pets (22). The animals for endoscopic studies were not given food for 16 h prior to examination.
Primary microbial isolation.
Up to four gastric biopsy samples were taken from each animal. One was used for rapid urease production testing (27); a positive result within 60 min was assumed to indicate the presence of Helicobacter spp., and culture was attempted with a second sample. The third sample was used for histology (unpublished data), and the fourth was used for electron microscopy. Biopsies for culture were handled as described earlier (19, 20). Both freshly prepared brain heart infusion (BHI) agar (Difco, Detroit, Mich.) and brucella agar (Oxoid, Basingstoke, United Kingdom), with cattle or horse blood and Skirrow’s antibiotic supplement (Oxoid), were used for isolation as described earlier (20). Plates were regularly checked for bacterial growth, and a few drops of BHI broth were added to the plates during the incubation period to avoid the drying of the media.
Primary identification of field isolates.
Strains were characterized by determining colonial and cellular morphology and the type of movement and by the urease reaction as described earlier (19, 26). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) whole-cell protein profiles were prepared and electrophoresed with a minigel system (running length, 4 cm) as described before (19), and the patterns were visually compared with those of type strains of relevant Helicobacter species.
In vivo electron micrographs.
Biopsy samples for electron micrographs were taken from 52 animals. The samples were fixed in 2.5% glutaraldehyde in Sörensen buffer containing 0.1 mmol of phosphate per liter (pH 7.3). After dehydration in acetone, the samples were embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and examined under a JEOL model 1200 EX electron microscope.
Extended phenotypic characterization.
Thirty-four representative strains of H. felis (n = 16; including three atypical H. felis strains), H. bizzozeronii (n = 10), H. salomonis (n = 6), and “F. rappini” (n = 2) (Table 1) were characterized with 65 phenotypic tests listed in a probability matrix for identifying campylobacters, helicobacters, and related taxa (44). Growth at 30°C and on buffered charcoal-yeast agar was not determined. In addition, spiral cell morphology (as determined by light-microscopic examination of Gram-stained bacterial films) was further distinguished into tightly or loosely coiled helical forms. Tests were performed with the recommended media by methods described previously (25, 39–43), with the following amendments. Nalidixic acid-, cephalothin-, metronidazole-, and carbenicillin-containing media were prepared by using filter-sterilized solutions prepared from the native antibiotic (obtained from Sigma Chemical Co. Ltd., Poole, England). Conditions of anaerobiosis, where needed, were produced in an anaerobic jar containing a palladium catalyst (Struers Kebo Lab, Copenhagen, Denmark) by applying four consecutive treatments of the gas replacement (anaerobic) method described by On and Holmes (41). All tolerance (growth) tests were performed with an inoculum size of ca. 109 CFU/ml and read after 3 and 7 days of incubation. The quality of all tests was examined by using appropriate control strains (37a).
TABLE 1.
Strains isolated in this study and reference strains
| Species | Straina | Received fromb | Source | Analysis performedc | Note |
|---|---|---|---|---|---|
| H. acinonychis | LMG 12684T, CCUG 29263T | CCUG | Canine gastric mucosa | c*, e | |
| H. bizzozeronii | CCUG 35545T, Storkis | Canine gastric mucosa | c*, d, e, f | ||
| H. bizzozeronii | CCUG 35546, 14 | Canine gastric mucosa | c*, d, e, f | ||
| H. bizzozeronii | Wiberg | Canine gastric mucosa | c*, d, e, g | ||
| H. bizzozeronii | 5F | Canine gastric mucosa | c*, d, e, f | ||
| H. bizzozeronii | 10 | Canine gastric mucosa | c*, d, e, f | ||
| H. bizzozeronii | 12A | Canine gastric mucosa | c*, d, e | ||
| H. bizzozeronii | Heydar | Canine gastric mucosa | c, d, e, g | ||
| H. bizzozeronii | Yrjala | Canine gastric mucosa | c, d, e, f | ||
| H. bizzozeronii | Emo | Canine gastric mucosa | d, e, f | ||
| H. bizzozeronii | Loko 21 | Canine gastric mucosa | c, d, e | ||
| H. bizzozeronii | 11AM | Canine gastric mucosa | d, e | ||
| H. bizzozeronii | Moppi | Canine gastric mucosa | e, g | ||
| H. bizzozeronii | Pinky | Canine gastric mucosa | e, g | ||
| H. bizzozeronii | Renny | Canine gastric mucosa | e, g | ||
| H. bizzozeronii | Bertta | Canine gastric mucosa | e, g | ||
| H. bizzozeronii | Roope | Canine gastric mucosa | e | ||
| H. bizzozeronii | Nummijärvi | Canine gastric mucosa | c, g | ||
| H. bizzozeronii | 9F | Canine gastric mucosa | c, f | ||
| H. bizzozeronii | 13 | Canine gastric mucosa | c | ||
| H. bizzozeronii | Loko 20 | Canine gastric mucosa | c, f, g | ||
| H. bizzozeronii | Rinne | Canine gastric mucosa | c, g | ||
| H. felis | CS 1, CCUG 28539T | CCUG | Feline gastric mucosa | c*, e, f | |
| H. felis | CS 5 | O’Rourke | Feline gastric mucosa | d, e | |
| H. felis | CS 6 | O’Rourke | Feline gastric mucosa | d, e | |
| H. felis | CS 7 | O’Rourke | Feline gastric mucosa | d, e | |
| H. felis | CS 8 | O’Rourke | Feline gastric mucosa | d, e | |
| H. felis | DS 1 | O’Rourke | Canine gastric mucosa | d, e | |
| H. felis | DS 3 | O’Rourke | Canine gastric mucosa | c*, d, e | |
| H. felis | DS 4, CCUG 28540 | CCUG | Canine gastric mucosa | c*, d, e, f | |
| H. felis | DS 5 | O’Rourke | Canine gastric mucosa | d, e | |
| H. felis | Dog 1, 1602 | Eaton | Canine gastric mucosa | d, e, f | |
| H. felis | Dog 2, 2301 | Eaton | Canine gastric mucosa | d, e | |
| H. felis | Dog 3, Dog 7 | Eaton | Canine gastric mucosa | c*, d, e, f | |
| H. felis | Into | Canine gastric mucosa | c, d, e, f | ||
| H. felis | Hellu | Canine gastric mucosa | c, d, e | ||
| H. felis | Kukka | Canine gastric mucosa | e, g | ||
| H. felis | Teppo | Canine gastric mucosa | e | ||
| H. felis | Loko 14 | Canine gastric mucosa | c, g | ||
| H. felis | Solanti | Canine gastric mucosa | c | ||
| H. felis | Tellu | Canine gastric mucosa | c | ||
| H. felis | Vilma | Canine gastric mucosa | c | ||
| H. felis | Cintti | Feline gastric mucosa | c, d, e, g | ||
| H. felis | Loki 13 | Feline gastric mucosa | c, f, g | ||
| H. felis | Fievel | Feline gastric mucosa | c, f, g | ||
| H. felis-H. bizzozeronii | Tuohimetsa | Canine gastric mucosa | c, d, e, f, g | Mixed culture | |
| H. felis-H. bizzozeronii | Jutta | Canine gastric mucosa | c, f, g | Mixed culture | |
| H. salomonis | InkinenT, CCUG 37845T | Canine gastric mucosa | c*, d, e, f, g | ||
| H. salomonis | 06A, CCUG 37848 | Canine gastric mucosa | c, e, f | ||
| H. salomonis | Alma | Canine gastric mucosa | c, f | ||
| H. salomonis | Elviira | Canine gastric mucosa | c, f | ||
| H. salomonis | Vilho | Canine gastric mucosa | c, d, e, f | ||
| H. salomonis | Mini | Canine gastric mucosa | c*, d, e, f, g | ||
| H. salomonis | Ko.K. III | Canine gastric mucosa | c, d, e, f | ||
| H. salomonis | Remu | Canine gastric mucosa | c, d, e, f, g | ||
| H. salomonis-H. bizzozeronii | Loko 18 | Canine gastric mucosa | c, d, e, f, g | Mixed culture | |
| “F. rappini” | CCUG 23435, ATCC 43879 | ATCC | Aborted sheep fetus | e | |
| “F. rappini” | Hilli | Canine gastric mucosa | c*, d, e, f | ||
| “F. rappini” | 19 | Canine gastric mucosa | c, d, e, f | ||
| H. mustelae | CCUG 25715T | CCUG | Ferret gastric mucosa | c*, e | |
| H. muridarum | CCUG 29262T, ATCC 49282T | CCUG | Murine intestinal mucosa | e | |
| H. nemestrinae | CCUG 32350T, ATCC 49396T | CCUG | Macaque gastric mucosa | e | |
| H. pylori | CCUG 17874T, ATCC 43504T | CCUG | Human gastric mucosa | e |
ATCC, American Type Culture Collection, Manassas, Va.; CCUG, Culture Collection, Department of Clinical Bacteriology, University of Göteborg, Göteborg, Sweden.
Own isolate unless indicated otherwise. Eaton, K. A. Eaton, Department of Veterinary Pathobiology, Ohio State University, Columbus; LMG, Culture Collection, Laboratorium voor Microbiologie, University of Ghent, Ghent, Belgium; O’Rourke, J. L. O’Rourke, School of Microbiology and Immunology, University of New South Wales, Sydney, Australia.
Ultrastructural analysis.
Electron microscopic examination of bacterial cell morphology was performed on 26 selected isolates (Table 1) by methods described previously (52).
Extraction, separation, and numerical analysis of whole-cell proteins.
The strains included in the highly standardized SDS-PAGE analysis are shown in Table 1. The strains were grown on Mueller-Hinton agar with 5% horse blood at 37°C in a microaerobic atmosphere for 72 h. Protein samples were prepared, separated by PAGE (running length, 9 cm), digitized, and subjected to comparative numerical analysis as described previously (47). Protein extraction and electrophoresis were performed as described earlier (47). Numerical analysis of the protein profiles was performed by using the GelCompar system, version 4.0 (Applied Maths, Kortijk, Belgium). The profiles were recorded and stored on a personal computer. The similarities between all pairs of traces were expressed by Pearson product moment correlation coefficients converted for convenience to percent values.
RESULTS
Primary isolation.
Fifty-one gastric Helicobacter isolates were obtained, of which 48 were of canine origin and 3 were isolated from cats. Three of the dog strains were lost during subcultivation and thus could not be examined in detail. The isolation rates of the canine and feline biopsies (as indexed to urease-positive biopsy samples) were 51 (48 of 95) and 14% (3 of 22), respectively. The primary growth appeared after 3 to 12 days of incubation as a thin spreading film; no single colonies were formed, and the primary area of growth was often very small. However, if air-dried plates were used, colonies could be observed, although the bacterial growth was of a poor quality. It was also noted that BHI, brucella, and Mueller-Hinton base agar media supported growth provided either cattle or horse blood was added (25a). The isolation rate was approximately the same throughout the study period (data not shown).
Of the taxa encountered in this study, H. felis proved the easiest to isolate. Relatively profuse growth was obtained after 4 to 7 days of incubation, and strains were readily subcultured. Similarly, H. salomonis also grew comparatively quickly and profusely on the primary plates, although examination of Gram-stained bacterial films indicated that cells transformed rapidly to coccoid forms and maintenance by subculture proved difficult. H. bizzozeronii isolates grew slowly, with primary growth observed within 4 to 12 days of incubation. The subculture of H. felis, H. bizzozeronii, and H. salomonis was expedited by the addition of a few drops of BHI broth to plate cultures. All commercial microaerobic atmospheres tested (gas generating kit model BR 56 with a palladium catalyst [Oxoid] and gas generating kit model BR 38 without the catalyst, [Oxoid]) as well as an evacuation and gas exchange system supported growth of these bacteria when the aforementioned subculturing procedures were employed. Additional hydrogen was not essential in order to obtain growth.
Four mixed cultures with two different Helicobacter spp. and one mixed culture with a Helicobacter sp. and a Campylobacter sp. were obtained. Two of these mixed cultures were recognized by examination of Gram-stained slide preparations and were subsequently purified. The first purified Helicobacter strain was contaminated with a Campylobacter-like organism, and the pure culture (H. bizzozeronii 13) was obtained after several subcultures on plates containing 100 IU of polymyxin B per ml (6). The second mixed isolate was originally “F. rappini”-H. bizzozeronii. As “F. rappini” strains show faster growth, the pure “F. rappini” strain (strain 19) was obtained from the edge of the culture. These two strains were then handled as pure cultures in the present study. The remaining three mixed cultures were revealed by the comparison of the protein pattern of the early culture with that of the later culture and were not successfully separated into pure cultures of the respective taxa (strain Jutta, H. bizzozeronii-H. felis; strain Tuohimetsa, H. bizzozeronii-H. felis; and strain Loko 18, H. bizzozeronii-H. salomonis).
Primary identification.
All fresh isolates were urease positive when first tested. Light-microscopic examination of Gram-stained bacterial films revealed that the isolates belonged to one of three morphological categories, corresponding to the taxa H. felis and H. bizzozeronii (tightly helical cells), H. salomonis (plump, less tightly coiled cells), and “F. rappini” (straight, cigar-shaped cells with tapering ends). Furthermore, two forms of motility were observed; a rapid, screw-like motion proved typical for H. felis, H. bizzozeronii, and “F. rappini” (with the unique cellular morphology of the last clearly distinct), while the movement of cells of H. salomonis strains was relatively slow and wavy. The visual examination of SDS-PAGE protein profiles with the minigel system clearly differentiated H. felis, H. bizzozeronii, and “F. rappini”. The differentiation of H. salomonis from H. bizzozeronii was more difficult with this method, and the final species designation was done with dot blot and quantitative DNA-DNA hybridization (26). The species distribution of canine isolates is shown in Table 2. Three strains were isolated from cats (two from healthy pets and one from a euthanized pet); these were all shown to be H. felis by the minigel SDS-PAGE system.
TABLE 2.
Dogs used in this study and the strains isolateda
| Type of dog | No. of dogs | No. of strains | % Isolation | No. of strains identified as:
|
No. of strains that were:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| H. felis | H. bizzozeronii | H. salomonis | “F. rappini” | Mixed culture | Unidentified | Lost | ||||
| Euthanized pet | 18 | 10 | 55 | 1 | 7 | 0 | 1 | 1 | 0 | 0 |
| Healthy pet | 37 | 16 | 43 | 1 | 7 | 3 | 0 | 2 | 2 | 1 |
| Gastrointestinal patient | 23 | 12 | 52 | 2 | 6 | 0 | 1 | 0 | 1 | 2 |
| Experimental | 17 | 10 | 59 | 4 | 1 | 5 | 0 | 0 | 0 | 0 |
| Total | 95 | 48 | 51 | 8 | 21 | 8 | 2 | 3 | 3 | 3 |
All biopsy samples were urease positive.
Ultrastructure of cultured strains.
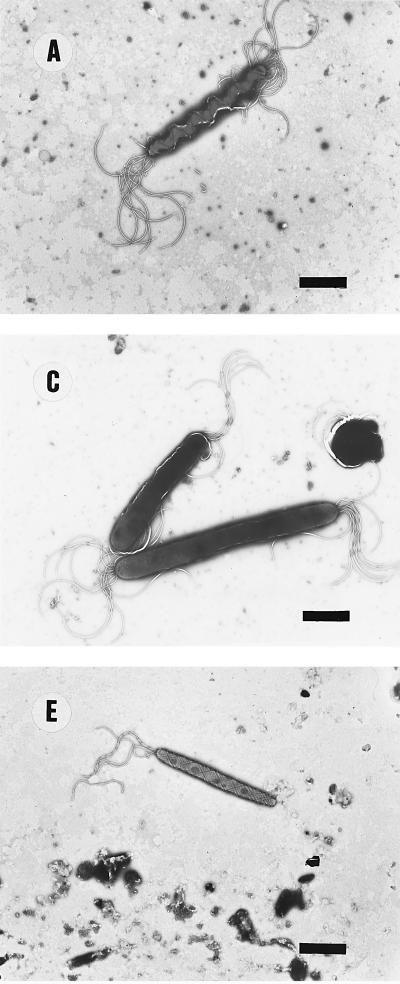
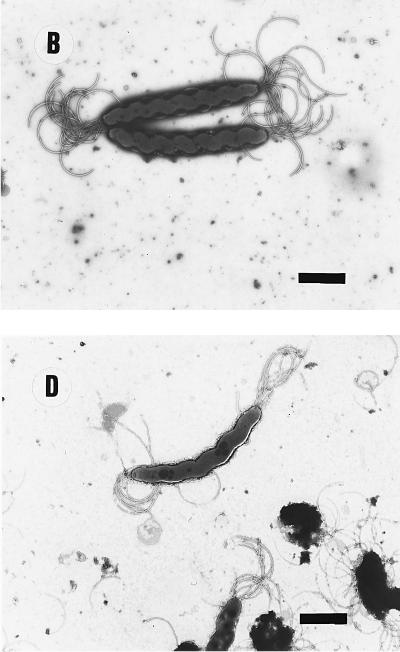
No significant infraspecific variation in cellular ultrastructure was observed in pure cultures of H. bizzozeronii (n = 8), H. salomonis (n = 8), or “F. rappini” (n = 2). Only the H. felis strains that were previously described as atypical by virtue of the absence of periplasmic fibrils (11) differed from other strains of this species. Typical examples of ultrastructural cell morphology of each species, and of the atypical H. felis strains, are reproduced in Fig. 1 and conform to descriptions given previously (11, 19, 26, 28, 29). In brief, typical H. felis cells were corkscrew-like, possessing one or two periplasmic fibrils; H. bizzozeronii strain cells were similar but slightly more helical, and no periplasmic fibrils were seen; H. salomonis cells were thicker and only slightly curved, while “F. rappini” cells were cigar-shaped organisms with tapering ends and had a remarkable net-like ultrastructure on the surface. The atypical H. felis strains closely resembled H. bizzozeronii in ultrastructure.
FIG. 1.
Electron micrographs of helicobacters and other organisms. (A) H. felis CCUG 28539T. Note the periplasmic fibrils around the cell body. (B) H. bizzozeronii CCUG 35545T. (C) H. salomonis 06A. The lower cell is about to divide, and a cell with a coccoid form is also visible. (D) Atypical H. felis cells where periplasmic fibrils are absent (Dog 1). The cell morphology closely resembles that of H. bizzozeronii (panel B). (E) “F. rappini” Hilli. Note the net-like surface and cigar-shape of the cell body. Bars, 2.3 μm.
In vivo electron micrographs.
Relatively few bacteria could be seen in electron micrographs prepared from tissue samples. Furthermore, these results did not always correlate with corresponding ultrastructural examinations of cultured bacteria. Moreover, only two types of organisms could be seen: either tightly coiled organisms without the periplasmic fibrils, resembling H. bizzozeronii or atypical H. felis (see above), or similar bacteria with the periplasmic fibrils, thus resembling H. felis. Thus, organisms resembling H. salomonis or “F. rappini” organisms were not detected. For seven animals (13%) where organisms resembling H. felis were seen, no culture was obtained. Also, for two animals H. felis cells were seen in tissue samples, yet H. bizzozeronii growth was obtained. H. felis-like cells were seen only in 12 samples (23%) and only as a small proportion of spiral organisms, most of which resembled H. bizzozeronii. In 36 animals (64%) H. bizzozeronii-like organisms were seen as the only colonizers. Conversely, although H. bizzozeronii-like strains were observed to be the sole colonizers of these animals, typical H. felis strains were isolated from four (7%) of these samples of which two were from cats.
Phenotypic characteristics.
Considerable difficulties in culturing bacteria for phenotypic testing were encountered with the methods used; consequently, two strains (strain 10 [H. bizzozeronii] and strain 06A [H. salomonis]) could not be tested. Similar problems were encountered when determining phenotypic properties of the cultured isolates. Initial studies using the recommended inoculum size for tolerance testing (106 CFU/ml [40]) proved unsuccessful, since many strains failed to grow even on the control medium (5% blood agar) with the aforementioned inoculum size (data not shown). Although results could be obtained by employing a significantly greater inoculum size (109 CFU/ml) and extending the incubation period for up to 7 days, the strains proved to be notably unreactive. No growth was observed on the following test media: unsupplemented nutrient agar; unsupplemented “Preston” (campylobacter charcoal-deoxycholate) medium; minimal medium; MacConkey agar; tyrosine and casein agars; and media containing 2, 3.5, or 4.0% NaCl, 0.1% KMnO4, 0.001% NaASO2, 0.02% or 0.05% safranin, 0.0005% crystal violet, 0.01% Janus green, 0.005% basic fuchsin, 0.1% sodium deoxycholate, and 0.02% pyronine. No growth on nutrient agar media containing 4 mg of metronidazole, 64 mg of cefoperazone, or 32 mg of carbenicillin per liter was noted. Strains did not grow at room temperature (18 to 22°C) or 25°C under microaerobic conditions or at 25 or 37°C aerobically. Strains did not hydrolyze hippurate or DNA, and reduction of selenite was not detected. Neither hydrogen sulfide nor acid in triple-sugar iron agar was produced. Bacterial growth did not exhibit any pigmentation, and pitting of agar media was not observed. All strains produced catalase. As a consequence of the difficulties encountered with culturing strains, nitrate reduction and α-hemolysis results could not be reliably determined for all isolates and are not presented here. Table 3 summarizes the results of those tests for which some differences between strains were noted. Tests clearly distinguishing H. bizzozeronii, H. felis, and H. salomonis were not identified in the scheme applied, although certain traits (namely distinctions in cell morphology, growth at 37°C on unsupplemented blood agar, and resistance to 5-fluorouracil [100 mg/liter]) provided a broad distinction between H. bizzozeronii or H. felis and H. salomonis. “F. rappini”-like strains were readily distinguished from the other taxa by being tolerant to 1.0, 1.5, and 2.0% bile, with other traits (elongated), loosely spiral cell morphology, alkaline phosphatase production, reduction of and growth on triphenyl-tetrazolium chloride medium, growth on potato starch medium) providing some additional discrimination from the other Helicobacter species isolated. In contrast with results obtained from freshly isolated strains, no urease activity was detected in four strains (“F. rappini”-like Hilli and H. bizzozeronii 11AM, Loko 21, and 12A), despite repeated examination and overnight incubation in the urease test medium.
TABLE 3.
Differential phenotypic characteristics of H. bizzozeronii, H. felis, H. salomonis, and “F. rappini”-like strains examined
| Characteristica | % of strains of indicated species positive for the characteristic
|
|||
|---|---|---|---|---|
| H. bizzozeronii (n = 10) | H. felis (n = 16) | H. salomonis (n = 6) | “F. rappini”-like (n = 2) | |
| Cell morphology | ||||
| Tightly coiled spiral | 78 | 67 | 0 | 0 |
| Loosely coiled spiral | 33 | 7 | 17 | 100 |
| Vibrioid/coccoid forms | 20 | 40 | 83 | 0 |
| Oxidase | 90 | 100 | 100 | 100 |
| Urease | 80 | 94 | 100 | 50 |
| Alkaline phosphatase | 50 | 62.5 | 50 | 100 |
| Indoxyl acetate hydrolysis | 20 | 25 | 17 | 0 |
| TTC reduction | 0 | 12.5 | 0 | 100 |
| Anaerobic growth on BA | 30 | 56 | 67 | 100 |
| Anaerobic growth on TMAO | 0 | 25 | 33 | 100 |
| Growth at 37°C | 90 | 100 | 67 | 100 |
| Growth at 42°C | 60 | 50 | 0 | 0 |
| Growth on: | ||||
| Potato starch medium | 0 | 6 | 17 | 99 |
| Lecithin medium | 0 | 12.5 | 0 | 0 |
| Tolerance to: | ||||
| 1, 1.5, and 2% bile | 0 | 0 | 0 | 100 |
| 1% glycine | 10 | 6 | 0 | 0 |
| 0.04% TTC | 0 | 12.5 | 0 | 100 |
| Nalidixic acid, 32 mg/liter | 0 | 6 | 0 | 0 |
| Cephalothin, 32 mg/liter | 10 | 19 | 50 | 100 |
| Metronidazole, 4 mg/liter | 0 | 12.5 | 17 | 0 |
| Carbenicillin, 32 mg/liter | 10 | 19 | 33 | 0 |
| Cefoperazone, 64 mg/liter | 0 | 6 | 0 | 0 |
| 5-Fluorouracil, 100 mg/liter | 80 | 75 | 17 | 100 |
| 0.05% sodium fluoride | 20 | 31 | 33 | 0 |
BA, blood agar; TMAO, trimethylamine N-oxide medium; TTC, triphenyl tetrazolium chloride.
Numerical analysis of protein profiles.
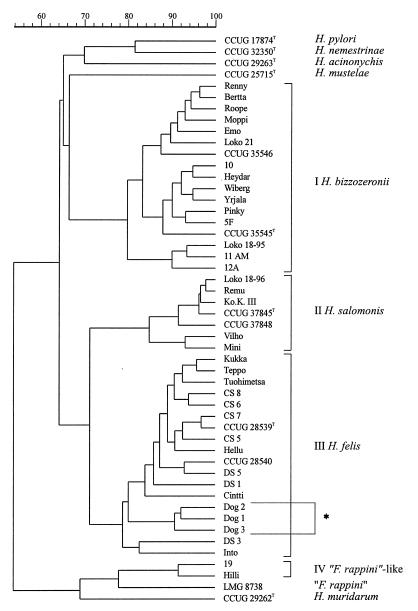
Duplicate protein extracts were prepared to check the reproducibility of the growth conditions and the preparation of the extracts. The correlation level between duplicate protein patterns was above 93% (data not shown). The whole-organism protein patterns of H. felis reference strains and of all of the strains isolated in the present study were compared with those of reference strains of other gastric helicobacters (i.e., H. acinonychis, H. mustelae, H. nemestrinae, and H. pylori) and of “F. rappini” CCUG 23435. A dendrogram illustrating the results of the numerical comparison of the whole-cell protein patterns of these strains is shown in Fig. 2; the dendrogram comprises four major clusters at the 77% similarity level. Clusters I and II comprise the H. bizzozeronii and H. salomonis strains, respectively, which group above similarity levels of 79.8 and 84.8%, respectively. Cluster III comprises the Australian reference strains of H. felis (29, 46), our own isolates, and the atypical H. felis strains without the periplasmic fibrils described by Eaton et al. (11). Cluster IV comprises the two isolates morphologically resembling “F. rappini.” The similarity level between the whole-cell protein patterns of the last two isolates is 91.6%, and this cluster is linked at the “F. rappini” reference strain, LMG 8738, at a similarity level of 77.9%. The type strains of H. acinonychis, H. mustelae, H. nemestrinae, and H. pylori each occupy distinct positions in the dendrogram. Figure 3 illustrates representative whole-cell protein patterns of the different groups of gastric isolates examined.
FIG. 2.
Dendrogram derived from the numerical analysis of the whole-cell protein patterns of all of the strains examined. Strains marked Loko 18-95 and Loko 18-96 represent the 1995 and 1996 subcultures of strain Loko 18. H. felis strains marked by an asterisk lack periplasmic fibrils. Roman numerals I through IV are the cluster numbers discussed in the text.
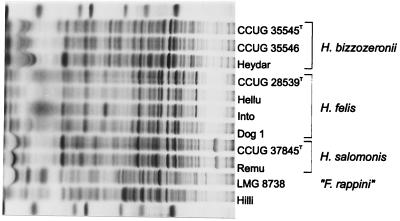
FIG. 3.
Whole-cell protein profiles of a representative selection of the strains examined. The molecular mass markers used (indicated in the bottom and top lanes) are (from left to right) lysozyme (14,500 Da), trypsin inhibitor (20,100 Da), trypsinogen (24,000 Da), carbonic anhydrase (29,000 Da), glyceraldehyde-3-phosphate dehydrogenase (36,000 Da), egg albumin (45,000 Da), and bovine albumin (66,000 Da).
The whole-cell protein patterns of the H. bizzozeronii strains are fairly homogeneous, with some variability primarily in the high-molecular-weight region (molecular weight > 60,000). The majority of the H. felis strains have very similar whole-cell protein patterns (Fig. 3). However, the strains without the periplasmic fibrils are characterized by the absence of two prominent bands which are present in all other strains (estimated molecular weights: 80,000 and 54,000; cf. the patterns of strains CCUG 28539T, Hellu, and Into with the pattern of strain Dog 1 in Fig. 3). Two additional strains have slightly aberrant patterns, characterized by an additional low-molecular-weight protein band of approximately 33,000 (Into) or by a slightly different size of the prominent protein band with an approximate molecular weight of 80,000 (DS 3). The patterns of the H. salomonis strains are very homogeneous and are typically characterized by an unusual prominent high-molecular-weight protein band (approximate size: 95,000; Fig. 3). Finally, the whole-cell protein patterns of the two “F. rappini”-like strains are similar to, but clearly different from, that of the “F. rappini” reference strain (LMG 8738) included in the analysis.
Two subcultures of the mixed culture of strain Loko 18 were included in the analysis. As illustrated in Fig. 2, the 1995 subculture of this strain (listed as Loko 18-95) was identified as H. bizzozeronii, while the 1996 subculture (listed as Loko 18-96) was identified as H. salomonis (Fig. 2). The species in the subculture of strain Tuohimetsa included in the present analysis was identified as H. felis (Fig. 2). It seems logical that the initial mixed cultures, which were dominated by the slow-growing H. bizzozeronii strains, were later dominated (after a considerable number of subcultivations in vitro) by the faster-growing H. salomonis and H. felis components of the mixed populations.
DISCUSSION
Isolation of canine and feline gastric Helicobacter spp.
Previous studies have demonstrated the presence of Helicobacter-like organisms in the gastric mucosa of virtually all adult cats and dogs (11, 22, 24, 36, 45). We have demonstrated that a variety of different Helicobacter species can be cultured from gastric biopsy samples of animals, particularly dogs, provided adequate isolation procedures are used. We attained Helicobacter isolation rates of 51% from dogs and 13.6% from cats, which exceed those reported previously (11, 36), and were successful in acquiring a diverse range of taxa, in contrast with other studies where only H. felis (7, 11, 23, 29), “F. rappini” (11), or intestinal helicobacters such as Helicobacter bilis (11) or Helicobacter pametensis (36) have been isolated in vitro. Clearly, the method used for primary isolation is critical. We examined only biopsies giving a positive urease reaction within 60 min, since there is considerable correlation between this and the actual number of helicobacters in the gastric mucosal biopsy (21, 30).
The main difficulties in obtaining bacterial growth were the long incubation period, high atmospheric humidity, and the use of moist, freshly prepared media, each of which contributed to the high level of contaminants encountered. We consider that fasting animals prior to biopsy is important in order to decrease the number of contaminating organisms, particularly since helicobacter growth was often first seen on the plate in a relatively small area, making subculture difficult. In addition, contamination of the primary plates of the cat biopsies was more severe than that found in comparable investigations of canine samples. This may partly explain the high proportion of culture failures for cat biopsies. However, H. pylori cells are often distributed unevenly within the human gastric mucosa (4), and it is conceivable that some of the culture failures encountered here were also due to similar phenomena, since only one biopsy sample was used for this purpose. Moreover, the differences noted here between the cell morphologies of the bacteria observed in vivo and those obtained in vitro may suggest that mixed populations of different taxa are much more common than the data allow us to conclude. However, it is equally possible that the cellular forms of some species cultured on artificial media differ from those the species display in the host environment, and such phenomena have been described for H. felis (29) and H. salomonis (26).
Although the methods used here yielded a considerably increased success rate of isolation (43.6% overall) compared with that of a previous study (10.2%) (11), 56.4% of biopsies in which helicobacters were indicated (i.e., rapidly urease positive) or observed failed to give positive results for culture. While the methods described here for isolation of these bacteria represent a considerable advance over those used previously, further improvements in culture conditions for these bacteria are still required. The development of noncultural methods of detection such as species-specific PCR tests would also be of benefit. Such methods were employed by Neiger et al. to infer that 78% of cats harbored “H. heilmannii” and none harbored H. felis (36). The suitability of genes such as the urease genes used by Neiger et al. for the differentiation of gastric helicobacters has not been determined, and therefore the sensitivity and specificity of such approaches are rightfully questioned. It is thus conceivable that the “H. heilmannii” isolates detected by Neiger et al. (36) represent misidentified strains of any of the other three taxa mentioned. Unfortunately, recent data show that the levels of 16S rDNA sequence similarity between H. felis, H. bizzozeronii, H. salomonis, and “H. heilmannii” are extremely high (26), and this widely used gene does not seem suitable as a target for species-specific PCR. In any case, available data strongly indicate the need for further work concerning the specificity and application of PCR assays for detecting the various Helicobacter spp. known to inhabit the gastric mucosa of domestic pets and humans (1, 17, 19, 26, 29).
Only three strains were isolated from cats, and all these were shown to be H. felis strains. It has been clearly observed in the early morphological studies comparing canine and feline gastric spiral organisms that the majority of feline gastric helicobacters are more tightly spiraled, thinner, and longer organisms than those from the dogs (55, 56). It may be that the tightly spiraled organisms without the periplasmic fibrils in cats represent a species different from H. bizzozeronii and our method is not adequate for isolating it. Further studies are needed to clarify this matter.
Identification of gastric Helicobacter spp.
The accurate identification of helicobacters and related organisms is essential in order to determine the prevalence and clinical significance of all taxa, although there are considerable difficulties associated with this process (37). Certainly the identification of strains to the species level was challenging in the present study. In primary identification tests, “F. rappini”-like isolates were readily distinguished from the other taxa encountered by virtue of their distinctive morphology, which was evident from both light and electron microscopy. Furthermore, H. salomonis isolates could be presumptively identified by their less helical cell morphology and unusually slow and sporadic motility (in contrast to H. bizzozeronii and H. felis), although the visual examination of protein patterns by using the minigel system proved a less useful means of identification. The unequivocal differentiation of H. bizzozeronii from H. felis was especially problematic. No clear differences were noted in primary identification tests. Moreover, the description of atypical H. felis strains lacking cellular periplasmic fibrils (11) invalidated this characteristic as an unequivocal means of distinguishing these two species, although all Finnish H. felis isolates proved typical in that respect. While useful, ultrastructural differences could not therefore be relied upon as a wholly accurate means of speciation, and the complex nature of the technique is not suited to routine use.
The phenotypic identification scheme used here has been found to provide effective discrimination between 11 of the 12 Helicobacter/“Flexispira” spp. tested (43, 44) and has also been used to identify field strains of the enteric species Helicobacter canis and Helicobacter pullorum (3, 38). In this study, considerable difficulties were encountered with simply cultivating the strains for further characterization, and these problems were reflected in the results obtained, since all strains were typified by their unreactivity. These data are consistent with the general difficulties associated with the isolation and culture of gastric helicobacters from domestic pets (11; this study). Nonetheless, it proved impossible to clearly differentiate the closely related species H. bizzozeronii, H. felis, and H. salomonis, although some useful traits for broadly distinguishing the last species from the first two taxa were noted (Table 3). Interestingly, urease was not detected in one “F. rappini”-like strain and three H. bizzozeronii isolates, suggesting a spontaneous loss of enzyme activity. This phenomenon has been described before for H. pylori (33) and H. mustelae (9).
The efficacy of highly standardized whole-cell protein analysis for identifying helicobacters and related organisms is well established (8, 37, 53) and was confirmed in the present study. All strains identified by quantitative or dot-blot DNA-DNA hybridization methods to the species level (26) were found to form discrete clusters after numerical analysis of the data. H. salomonis, H. bizzozeronii, and H. felis were all readily recognizable. The three H. felis strains without periplasmic fibrils clustered among the other H. felis strains, although two prominent protein bands were absent. The whole-cell protein analysis confirms the identification of these strains as H. felis (11, 26) but highlights their aberrant nature. Therefore, we strongly recommend the use of highly standardized SDS-PAGE as the means of species identification for gastric spiral Helicobacter spp.
The identity of the “F. rappini”-like strains (strains 19 and Hilli) is undetermined. Both strains have a typical Helicobacter whole-cell protein pattern which shares some similarities with that of the “F. rappini” reference strain examined (LMG 8738; also ATCC 43879). Strain LMG 8738 is a human “F. rappini” reference strain isolated by Archer et al. in 1988 (2). Later studies revealed significant heterogeneity among isolates tentatively classified as “F. rappini,” and novel Helicobacter species with similar ultrastructural features have been described (H. bilis and H. trogontum [16, 35]). H. bilis and H. trogontum are, however, intestinal helicobacters isolated from rodents, while strains 19 and Hilli are canine gastric strains. Our data indicate that strains 19 and Hilli do not belong to the same species as strain LMG 8738; several phenotypic differences (namely growth at 42°C, anaerobic growth, and growth on starch and media containing bile or triphenyl-tetrazolium chloride) are also evident (this study; 44). The relationship of strains 19 and Hilli to H. bilis, H. trogontum, and other helicobacters is under investigation.
The prospect of strain misidentification due to mixed cultures is evident from the examples described in this study (Fig. 2), although this problem is not easily solved. Several species may coexist in the gastric tracts of domestic pets (19, 26, 29; this study), and the bacterial growth of many of these helicobacters shows a tendency to swarm. This is strikingly illustrated by the differences in morphology between the organisms seen in in vivo electron micrographs and the culture isolates. Campylobacter contaminants have been noted in primary cultures of H. felis (29), although the differential susceptibilities of helicobacters and campylobacters to polymyxin B (6, 25a) can be exploited to eliminate campylobacter contamination. Conversely, no tolerance tests to differentiate between the Helicobacter spp. studied were found in the present study, and no recommendations for selective media can be made at present. Logically, repeated subcultivation of these mixed cultures and transfer of the strains after a relatively short incubation period will favor growth of the less fastidious strains. In our experience, an apparently pure “F. rappini”-like strain was recovered from a mixture of “F. rappini” and H. bizzozeronii strains and H. salomonis or H. felis strains seemed to become the dominant strains in mixtures which were initially dominated by H. bizzozeronii strains (strains Jutta, Tuohimetsa, and Loko 18). The prospect of a mixed culture must be considered when examining suspect helicobacter growth from a canine or feline gastric biopsy. Consequently, the cell morphology, motility, and protein profile of any isolate should be carefully scrutinized for anomalies.
Prevalence and significance of gastric helicobacters in domestic pets.
We isolated only H. felis from feline gastric biopsies. By contrast, H. bizzozeronii, H. felis, H. salomonis, and “F. rappini” were obtained in 55.6, 22.2, 22.2, and 4.4%, respectively, of canine biopsies; mixed cultures were obtained in a total of 8.8% of these biopsies. Previous studies of the ecology of different Helicobacter species in the gastric mucosa of domestic carnivores are difficult to interpret, especially in view of recent developments in our understanding of the taxonomic diversity of these bacteria (11, 19, 26). Our data clearly indicate that several distinct species colonize the canine gastric mucosa and may also coexist with related taxa.
However, the clinical significance of these bacteria is unclear. Mild-to-moderate gastritis in infected animals is seen without any gastrointestinal signs (11, 22), and gastritis has also been observed without bacterial association (36). Moreover, it has been difficult to draw any conclusions from these studies as almost all dogs and cats are infected and the number of negative controls is low in a natural population (11, 22). However, an experimental infection of gnotobiotic beagles with H. felis caused gastritis in the animals (30). Similarly, natural H. felis infection has been suspected as a cause of severe gastrointestinal signs (48). No such data are available regarding H. bizzozeronii, H. salomonis, or “F. rappini,” but it is feasible that intraspecific variation in pathogenic potential might explain the variance in the severity of gastritis seen among animals. Further work is required to clarify this issue.
The zoonotic potential of gastric helicobacters has been the subject of considerable interest, especially since the isolation of H. pylori from cats (18). Although subsequent studies have indicated that cats do not represent a significant reservoir for human H. pylori infection (11, 13), the issue concerning “H. heilmannii” (also known as “G. hominis”) is far less certain. Several reports describe “H. heilmannii” infection as a possible zoonosis (34, 51). However, “H. heilmannii” is ultrastructurally indistinguishable from H. bizzozeronii and the atypical H. felis strains described by Eaton et al. (11) in vivo, and all these taxa have a complex relationship by 16S rRNA sequence analysis (26). Clearly, it is crucial to accurately determine the taxonomic position of “H. heilmannii” to properly evaluate its zoonotic potential, but such studies have been hindered by the failure of most workers to culture the organism (34, 51). It is possible that the methods described in this report for the culture of canine and feline helicobacters could be used to isolate human “H. heilmannii” strains, facilitating the necessary investigations. In this respect, it is encouraging to note that one such strain has been described (1), and we are pursuing comparative taxonomic studies to clarify this important issue.
ACKNOWLEDGMENTS
We thank Urszula Hirvi and Dirk Dewettinck for excellent technical assistance. We are grateful to all of the depositors of the strains listed in Table 1.
Katri Jalava has been supported by the young scientists award of Emil Aaltosen säätiö and the Finnish Academy of Science. P.V. is indebted to the Fund for Scientific Research-Flanders (Belgium) for a position as a postdoctoral fellow.
REFERENCES
- 1.Andersen L P, Norgaard A, Holck S, Blom J, Elsborg L. Isolation of “Helicobacter heilmannii” from human tissue. Eur J Clin Microbiol Infect Dis. 1996;15:95–96. doi: 10.1007/BF01586196. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Archer J R, Romero S, Ritchie A E, Hamacher M E, Steiner B M, Bryner J H, Schell R F. Characterization of an unclassified microaerophilic bacterium associated with gastroenteritis. J Clin Microbiol. 1988;26:101–105. doi: 10.1128/jcm.26.1.101-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atabay H I, Corry J E L, On S L W. Identification of unusual Campylobacter-like organisms in poultry products as Helicobacter pullorum. J Appl Microbiol. 1998;84:1017–1024. doi: 10.1046/j.1365-2672.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 4.Bayerdorffer E, Oertel H, Lehn N, Kasper G, Mannes G A, Sauerbruch T, Stolte M. Topographic association between active gastritis and Campylobacter pylori colonisation. J Clin Pathol. 1989;42:834–839. doi: 10.1136/jcp.42.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronsdon M A, Goodwin C S, Sly L I, Chilvers T, Schoenknecht F D. Helicobacter nemestrinae sp. nov., a spiral bacterium found in the stomach of a pigtailed macaque (Macaca nemestrina) Int J Syst Bacteriol. 1991;41:148–153. doi: 10.1099/00207713-41-1-148. [DOI] [PubMed] [Google Scholar]
- 6.Burnens A P, Nicolet J. Three supplementary diagnostic tests for Campylobacter species and related organisms. J Clin Microbiol. 1993;31:708–710. doi: 10.1128/jcm.31.3.708-710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattoli G, Zanoni R, Benazzi C, Della Salda L, Serraino A, Sanguinetti V. Isolation of Helicobacter felis from dogs in Italy. In: Newell D, et al., editors. Proceedings of Campylobacters, Helicobacters and related organisms. New York, N.Y: Plenum Press; 1996. pp. 341–343. [Google Scholar]
- 8.Costas M, On S L W, Owen R J, Lopez-Urquijo B, Lastovica A J. Differentiation of Helicobacter species by numerical analysis of their one-dimensional electrophoretic protein patterns. Syst Appl Microbiol. 1993;16:396–404. [Google Scholar]
- 9.Costas M, Owen R J, Morgan D D, Goodwin C S. Loss of urease activity in Helicobacter mustelae. Lett Appl Microbiol. 1991;14:260–264. [Google Scholar]
- 10.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K A, Dewhirst F E, Radin M J, Fox J G, Paster B J, Krakowka S, Morgan D R. Helicobacter acinonyx sp. nov., isolated from cheetahs with gastritis. Int J Syst Bacteriol. 1993;43:99–106. doi: 10.1099/00207713-43-1-99. [DOI] [PubMed] [Google Scholar]
- 13.El-Zaatari F A, Woo J S, Badr A, Osato M S, Serna H, Lichtenberger L M, Genta R M, Graham D Y. Failure to isolate Helicobacter pylori from stray cats indicates that H. pylori in cats may be an anthroponosis—an animal infection with a human pathogen. J Med Microbiol. 1997;46:372–376. doi: 10.1099/00222615-46-5-372. [DOI] [PubMed] [Google Scholar]
- 14.Fox J G, Lee A. The role of Helicobacter species in newly recognized tract diseases of animals. Lab Anim Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- 15.Fox J G, Taylor N S, Edmonds P, Brenner D J. Campylobacter pylori subsp. mustelae subsp. nov. isolated from the gastric mucosa of ferrets (Mustela putorius furo), and an emended description of Campylobacter pylori. Int J Syst Bacteriol. 1988;38:367–370. [Google Scholar]
- 16.Fox J G, Yan L L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germani Y, Dauga C, Duval P, Huerre M, Levy M, Pialox G, Sansonetti P, Grimont P A D. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H. felis in a human gastric biopsy. Res Microbiol. 1997;148:315–326. doi: 10.1016/S0923-2508(97)81587-2. [DOI] [PubMed] [Google Scholar]
- 18.Handt L K, Fox J G, Dewhirst F E, Fraser G J, Paster B J, Yan L L, Rozimiarek H, Rufo R, Stalis I H. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hänninen M-L, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996;46:160–166. doi: 10.1099/00207713-46-1-160. [DOI] [PubMed] [Google Scholar]
- 20.Hänninen M-L, Jalava K, Saari S, Happonen I, Westermarck E. Culture of “Gastrospirillum” from gastric biopsies of dogs. Eur J Clin Microbiol Infect Dis. 1995;14:145. doi: 10.1007/BF02111876. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 21.Happonen I, Saari S, Castren L, Tyni O, Hänninen M-L, Westermarck E. Comparison of diagnostic methods for detecting gastric Helicobacter-like organisms in dogs and cats. J Comp Pathol. 1996;115:117–127. doi: 10.1016/s0021-9975(96)80034-x. [DOI] [PubMed] [Google Scholar]
- 22.Happonen I, Saari S, Castren L, Tyni O, Hänninen M-L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. J Vet Med Assoc. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 23.Haziroglu R, Diker S, Guvenc T, Kul O. Canine gastritis associated with Helicobacter felis. Dtsch Tieräerztl Wochenschr. 1995;102:474–476. [PubMed] [Google Scholar]
- 24.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 25.Holmes B, On S L W, Ganner M, Costas M. Some new applications of probabilistic identification. In: Schindler J, editor. Proceedings of the 1992 Conference on Taxonomy and Automated Identification of Bacteria. Prague, Czechoslovakia: Czechoslovak Society for Microbiology; 1992. pp. 6–9. [Google Scholar]
- 25a.Jalava, K. Unpublished data.
- 26.Jalava K, Kaartinen M, Utriainen M, Happonen I, Hänninen M-L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997;47:975–982. doi: 10.1099/00207713-47-4-975. [DOI] [PubMed] [Google Scholar]
- 27.Katelaris P H, Lowe D G, Norbu P, Farthing J G. Field evaluation of a rapid, simple and inexpensive urease test for the detection of Helicobacter pylori. J Gastroenterol Hepatol. 1992;7:569–571. doi: 10.1111/j.1440-1746.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirkbride C A, Gates C E, Collins J E, Ritchie A E. Ovine abortion associated with an anaerobic bacterium. J Am Vet Med Assoc. 1985;186:789–791. [PubMed] [Google Scholar]
- 29.Lee A, Hazel S L, O’Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee A, Krakowka S, Fox J G, Otto G, Eaton K A, Murphy J C. Role of H. felis in chronic canine gastritis. Vet Pathol. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Phillips M W, O’Rourke J L, Paster B J, Dewhirst F E, Fraser G J, Fox J G, Sly L I, Romaniuk P J, Trust T J, Kouprach S. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int J Syst Bacteriol. 1992;42:27–36. doi: 10.1099/00207713-42-1-27. [DOI] [PubMed] [Google Scholar]
- 32.Lockard V G, Boler R K. Ultrastructure of a spiraled microorganism in the gastric mucosa of dogs. Am J Vet Res. 1970;31:1453–1462. [PubMed] [Google Scholar]
- 33.Majewski S I H, Goodwin C S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988;157:465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- 34.McNulty C A M, Dent J C, Curry A, Uff J S, Ford G A, Gear M W L, Wilkinson S P. New spiral bacterium in the gastric mucosa. J Clin Pathol. 1989;42:585–591. doi: 10.1136/jcp.42.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendes E N, Queiroz D M, Dewhirst F E, Paster B J, Moura S B, Fox J G. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int J Syst Bacteriol. 1996;46:916–921. doi: 10.1099/00207713-46-4-916. [DOI] [PubMed] [Google Scholar]
- 36.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.On S L W. Identification methods for campylobacters, helicobacters, and related organisms. Clin Microbiol Rev. 1996;9:405–422. doi: 10.1128/cmr.9.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.On, S. L. W. Unpublished data.
- 38.On, S. L. W., H. I. Atabay, and C. S. Harrington. Evaluation of a probability matrix for identifying campylobacteria. In A. Lastovica, D. G. Newell, and E. E. Lastovica (ed.), Campylobacter IX. Proceedings of the 9th International Workshop on Campylobacter, Helicobacter and Related Organisms, in press.
- 39.On S L W, Costas M, Holmes B. Classification and identification of Campylobacter sputorum using numerical analyses of phenotypic tests and of whole-cell protein profiles. Syst Appl Microbiol. 1994;17:543–553. [Google Scholar]
- 40.On S L W, Holmes B. Effect of inoculum size on the phenotypic characterization of Campylobacter species. J Clin Microbiol. 1991;29:923–926. doi: 10.1128/jcm.29.5.923-926.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.On S L W, Holmes B. Reproducibility of tolerance tests that are useful in the identification of campylobacteria. J Clin Microbiol. 1991;29:1785–1788. doi: 10.1128/jcm.29.9.1785-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.On S L W, Holmes B. Assessment of enzyme detection tests useful in identification of campylobacteria. J Clin Microbiol. 1992;30:746–749. doi: 10.1128/jcm.30.3.746-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.On S L W, Holmes B. Classification and identification of campylobacters, helicobacters and allied taxa by numerical analysis of phenotypic tests. Syst Appl Microbiol. 1995;18:374–390. [Google Scholar]
- 44.On S L W, Holmes B, Sackin M J. A probability matrix for the identification of campylobacters, helicobacters and allied taxa. J Appl Bacteriol. 1996;81:425–432. doi: 10.1111/j.1365-2672.1996.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 45.Otto G, Hazell S H, Fox J G, Howlett C R, Murphy J C, O’Rourke J L, Lee A. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol. 1994;32:1043–1049. doi: 10.1128/jcm.32.4.1043-1049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paster B J, Lee A, Fox J G, Dewhirst F E, Tordoff L A, Fraser G J, O’Rourke J L, Taylor N S, Ferrero R. Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int J Syst Bacteriol. 1991;41:31–38. doi: 10.1099/00207713-41-1-31. [DOI] [PubMed] [Google Scholar]
- 47.Pot B, Vandamme P, Kersters K. Analysis of electrophoretic whole-organism protein fingerprints. In: Goodfellow M, O’Donnell A G, editors. Modern microbial methods. Chemical methods in prokaryotic systematics. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1994. pp. 493–521. [Google Scholar]
- 48.Salda D L, Cattoli G, Zanoni R, Sanguinetti V, Serraino A, Banazzi C. Isolation of Helicobacter felis from naturally dead kennel dogs in Italy, abstr. 124. Eur J Vet Pathol Suppl. 1996;2:39. [Google Scholar]
- 49.Salomon H. Ueber das Spirillum des Säugetiermagens und sein Verhalten zu den Belegzellen. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1898;19:422–441. [Google Scholar]
- 50.Schauer D B, Ghori N, Falkow S. Isolation and characterization of “Flexispira rappini” from laboratory mice. J Clin Microbiol. 1993;31:2709–2714. doi: 10.1128/jcm.31.10.2709-2714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solnick J V, O’Rourke J, Lee A, Paster B J, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- 52.Utriainen M, Jalava K, Sukura A, Hänninen M-L. Morphological diversity of cultured canine gastric Helicobacter spp. Comp Immunol Microbiol Infect Dis. 1997;20:285–297. doi: 10.1016/s0147-9571(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 53.Vandamme P, Falsen E, Hoste R, Segers B, Tytgat P, DeLey J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991;41:88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]
- 54.Warren B J, Marshall J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 55.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla in the fundic glands of dogs and cats. Am J Vet Res. 1958;19:677–680. [PubMed] [Google Scholar]
- 56.Weber A F, Schmittdiel E F. Electron microscopic and bacteriologic studies of spirilla isolated from the fundic stomachs of cats and dogs. Am J Vet Res. 1962;23:422–427. [PubMed] [Google Scholar]
- 57.Wegmann W, Achwanden M, Schaub N, Aenishänslin W, Gyr K. Gastrospirillum hominis-assoziierte gastritis—eine Zoonose? Schweiz Med Wochenschr. 1991;121:245–254. [PubMed] [Google Scholar]