Abstract
CD19-directed CAR T-cell therapy with brexucabtagene autoleucel (brexu-cel) has substantially improved treatment outcomes for patients with relapsed/refractory mantle cell lymphoma (r/r MCL). Prolonged cytopenias and infections represent common and clinically relevant side effects. In this multicenter observational study, we describe cytopenias and infections in 103 r/r MCL patients receiving brexu-cel. Furthermore, we report associations between the baseline CAR-HEMATOTOX (HT) score and toxicity events, non-relapse mortality (NRM), and progression-free/overall survival (PFS/OS). At lymphodepletion, 56 patients were HTlow (score 0–1) while 47 patients were HThigh (score ≥2). The HThigh cohort exhibited prolonged neutropenia (median 14 vs. 6 days, p<0.001) and an increased rate of severe infections (30% vs. 5%, p=0.001). Overall, 1-year NRM was 10.4%, primarily attributed to infections, and differed by baseline HT score (high vs. low: 17% vs. 4.6%, p=0.04). HThigh patients experienced inferior 90-day complete response rate (68% vs. 93%, p=0.002), PFS (median 9 months vs. not-reached, p<0.0001) and OS (median 26 months vs. not-reached, p<0.0001). Multivariable analyses showed that high HT scores were independently associated with severe hematotoxicity, infections, and poor PFS/OS. In conclusion, infections and hematotoxicity are common after brexu-cel and contribute to NRM. The baseline HT score identified patients at increased risk of poor treatment outcomes.
Keywords: Chimeric Antigen Receptor T-cell Therapy, Mantle Cell Lymphoma, Hematotoxicity, Infections, Risk Factors
Graphical Abstract
Introduction
Mantle cell lymphoma (MCL) represents a genetically and clinically heterogenous B-cell lymphoma that can present both in the form of indolent cases that do not require therapeutic intervention for years, to highly aggressive disease with a very limited prognosis.1,2 While Bruton’s tyrosine kinase (BTK) inhibitors have improved clinical outcomes for both older and younger patient populations,3,4 relapsed and/or refractory (r/r) MCL patients typically have a poor prognosis. Recently, CD19-directed chimeric antigen receptor (CAR) T-cell therapy with brexucabtagene autoleucel (brexu-cel) has broadened the armamentarium for r/r MCL patients with promising response rates both in clinical studies and the real-world setting.5–8 Even though a substantial number of patients achieve long-term remissions, CAR T-cell therapy is accompanied by a unique spectrum of side effects including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).9–11 However, the most frequent grade ≥3 side effect is hematological toxicity, which may present in the form of profound and/or long-lasting cytopenias.12–15 Importantly, such sustained cellular immunosuppression predisposes for severe infectious complications.16–18 Indeed, fatal infections now represent the driving determinant of non-relapse mortality (NRM), mainly due to advances in the management of CRS and ICANS (e.g. tocilizumab, anakinra, early glucocorticoids).19–22
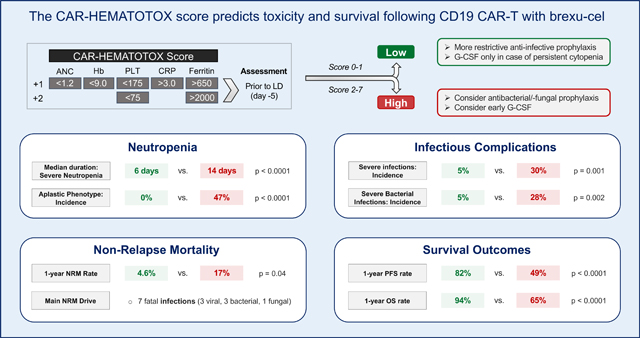
The occurrence of cytopenia long after lymphodepletion makes it unlikely that hematotoxicity results as a direct effect of the applied cytotoxic chemotherapy alone.23 Patients often exhibit biphasic count recovery with recurrent dips of peripheral blood counts during routine follow-up.12,23 In qualitative terms, post-CAR-T neutrophil recovery typically follows three unique trajectories: quick vs. intermittent vs. aplastic.12 While the underyling pathophysiology still remains unclear, high-grade CRS together with the associated cytokine patterns (particularly IL-6) have been linked to more severe manifestations of hematotoxicity.13,24 Furthermore, we previously demonstrated that immune dysregulation and the baseline inflammatory state, as reflected by serum CRP and ferritin levels, were particularly relevant for the development of prolonged neutropenia.25 When combined with baseline cytopenia, the resulting CAR-HEMATOTOX (HT) score identified patients at high risk for CAR-T-related hematotoxicity in a r/r large B-cell lymphoma (LBCL) patient cohort.12 In a follow-up study, the HT score also risk-stratified for severe infections and adverse treatment outcomes.20
Overall, detailed reporting on cytopenias and infectious complications following brexu-cel remains scarce for the real-world setting and detailed analyses outlining not only the quantity but also quality of cytopenias have not been performed to date. Furthermore, no early risk-stratification system for hematological toxicity, infections, and survival outcomes has been validated for r/r MCL patients. In this study, we therefore aimed to broadly characterize toxicity and survival in a large multi-center cohort, and to specifically investigate the impact of the HT score on these outcomes.
Methods
Patients and data collection
All r/r MCL patients receiving brexu-cel between December 2015 and July 2022 across eight international CAR-T centers were included. Toxicity and survival outcomes were assessed in 103 patients, including 89 patients treated in a standard-of-care setting and 14 patients treated on clinical trial (NCT02601313). Lymphodepleting chemotherapy with fludarabine and cyclophosphamide was administered according to the manufacturers’ instructions.5 Clinical metadata were extracted from medical records and databases with IRB approval (Supplemental Methods).
CAR-HEMATOTOX
The score was preferentially calculated on the day of lymphodepletion (day −5) using the German Lymphoma Alliance (GLA) online calculator: https://www.german-lymphoma-alliance.de/Scores.html. A leniency period of up to three days for laboratory markers was provided (Supplemental Methods).12
Defining hematological toxicity and neutrophil recovery phenotypes
Severe anemia was defined as hemoglobin <8 g/dL or requiring transfusion. Severe thrombocytopenia was defined as a platelet count <50 G/L. Neutropenia was defined based on the joint American Society of Clinical Oncology/Infectious Diseases Society of America (ASCO/IDSA) consensus guidelines26 for cancer-related infection risk: severe (absolute neutrophil count [ANC] <500/μL), profound (ANC <100/μL) and protracted (lasting ≥7 days) neutropenia. For each patient, we determined the total cumulative duration of severe neutropenia between days 0 and +60, as previously described.12 The patients were allocated to a phenotype of neutrophil recovery according to the following definitions:
Quick: sustained neutrophil recovery without a second dip below an ANC <1000/μL.
Intermittent: neutrophil recovery (ANC >1500/μl) followed by a second dip with an ANC <1000/μL after day 21.
Aplastic: continuous severe neutropenia (ANC <500/μL) ≥14 days.
Toxicity and infection grading
American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria were applied for CRS and ICANS grading.27 Toxicity management followed institutional guidelines.20,28 Infections were studied until day +90 after brexu-cel. Each infection event was defined as bacterial, viral, or fungal based on microbiologic or histopathologic data, or as a clinical syndrome of infection (e.g., pneumonia, cellulitis, cystitis) based on retrospective chart review. Infection onset was defined as the day of the diagnostic test. Clinical infection source was based on the combination of clinical signs and symptoms, microbiologic isolates, and radiographic findings. Neutropenic fever without clinical symptoms and/or microbiologic data was not counted as an infection event. Infections were graded on a 5-grade scale as mild, moderate, severe, life-threatening, or fatal as previously described.16,20,29
Clinical outcomes
Efficacy outcomes were assessed according to Lugano Criteria.30 Kaplan-Meier estimates for PFS and OS were calculated from time of CAR-T infusion. HT score groups (high vs. low) were compared by log-rank test. Follow-up was calculated using the reverse Kaplan-Meier method. NRM was defined as death following brexu-cel without evidence of relapse or progression. Length of hospitalization was determined between admission and the first discharge from the hospital.
Statistical Considerations
Univariate and multivariable analyses of pre- and post-CAR-T factors were performed using a Cox proportional hazards model for PFS/OS and binary logistic regression analysis studying the aplastic phenotype and severe infection as binary outcomes. All covariates with a p<0.2 on univariate analysis were included in the multivariable models. Receiver operating characteristic (ROC) analysis was performed to assess test characteristics of the HT score. Associations between continuous variables were analyzed using the Spearman correlation coefficient (r). Statistical significance between groups was explored by non-parametric Mann-Whitney test for continuous variables and Fisher’s exact test for comparison of percentages. Statistical analysis and data visualization was performed using GraphPad Prism (v9.0), SPSS (IBM, v26.0), and R Statistical Software (v4.1.2).
Results
Patient characteristics and disposition
We assessed the incidence and clinical features of CAR-T mediated toxicity and survival outcomes in 103 r/r MCL patients receiving brexu-cel at a median follow up of 15.4 months. An overview of patient demographic and laboratory characteristics is provided in Table S1. The median age was 66 years (range 49–89); median ECOG performance status (PS) was 1 (interquartile range [IQR] 0–1). Patients received a median of 3 prior treatment lines (IQR 2–4), including 32% with prior autologous stem cell transplantation (ASCT), 41% with prior bendamustine therapy, and 16% with bendamustine exposure in the last 6 months prior to CAR-T infusion. Of interest, the majority of patients received bridging therapy (79%). In terms of disease characteristics, blastoid or pleomorphic histology was noted in 41 patients (40%), while 77 patients (75%) exhibited an intermediate or high-risk simplified MCL international prognostic index (sMIPI) score. In evaluable patients, an elevated Ki-67% proliferation index (≥30%) was observed in 78% of cases, while 42% of patients had a TP53 alteration. Underlying bone marrow (BM) involvement was observed in 37% of patients, while CNS involvement was noted in 5 cases.
Our study cohort comprised of 56 HTlow (score 0–1) and 47 HThigh (score 2–7) patients. The median HT score was 1 (IQR 1–2). When comparing the two risk groups, we did not observe a difference in terms of baseline ECOG, age/gender/race distribution, geographic region, kidney function, utilization of bridging therapy, and prior allogeneic or autologous SCT (Table S1). However, we noted an increased fraction of HThigh patients with intermediate or high sMIPI scores (86% vs. 66%, p=0.04), an elevated Ki-67 proliferation index (88% vs. 69%, p=0.04), and the adverse risk blastoid or pleomorphic disease histology (51% vs. 30%, p=0.04). In evaluable patients, a non-significant difference was noted for the presence of complex karyotype in HThigh vs. HTlow patients (50% vs. 24%, p=0.065). Furthermore, the high-risk group more frequently observed underlying BM infiltration on last biopsy prior to CAR-T (49% vs. 27%, p=0.025), and displayed higher serum LDH levels at lymphodepletion (median 255 vs. 207 U/L, p=0.02). As expected due to the composition of the score, the HThigh group was characterized by high levels of the serum inflammatory markers CRP (median 3.1 mg/dL, 95% confidence interval [CI] 1.1–4.0 mg/dL) and ferritin (median 324 ng/mL, 95% CI 228–645 ng/mL). These patients also exhibited significant baseline cytopenia with a median hemoglobin of 10.1 g/dL (95% CI 9.3–10.6 g/dL), median platelet count of 101 G/L (95% CI 79–129 G/L) and a median ANC of 1640/μL (95% CI 1120–2480/μL).
Overall incidence of early and late cytopenia and quality of neutrophil recovery
The overall incidence of hematological toxicity was high in our cohort. The median duration of severe neutropenia (ANC <500/μL) was 8 days (95% CI 7–9 days), and 91 patients (88%) dropped their counts below this threshold during the first 30 days following brexu-cel infusion. In 61 patients (59%), we observed severe neutropenia lasting ≥7 days (Table 1). Additionally, 28 patients (27%) exhibited severe neutropenia between days +31 to +100. Profound neutropenia (ANC <100/μL) during the first 100 days was noted in 66 patients (64%). This included 26% of patients with protracted, profound neutropenia (ANC <100/μL lasting ≥7 days) – a risk category strongly associated with infectious complications across disease entities and cancer treatments.26 Furthermore, 48 patients (47%) developed prolonged neutropenia, defined as an ANC <1000/μL after day +21. The distribution of neutrophil recovery phenotypes was 41% for quick, 38% for intermittent, and 21% for aplastic. Severe thrombocytopenia (platelet count <50 G/L) and anemia (Hb <8 g/dL or requiring packed red blood cell [pRBC] transfusion) were observed in 57% and 51% of patients, respectively. Platelet and pRBC transfusions were applied in 34% and 41% of patients during the first 30 days. Growth factors were administered in 58 patients (56%) after a median of 14 days. Thrombopoietin (TPO) agonists were applied in 8 patients. Only one patient received an autologous stem cell boost.
Table 1. Hematotoxicity and Management.
Overview of cytopenia incidence rates and concomitant management strategies during the first 100 days after brexu-cel infusion stratified by CAR-HEMATOTOX score. P-values determined by Mann-Whitney test for continuous variables and Fisher’s exact tests for categorical variables.
| Characteristic | All Patients (n=103) | CAR-HEMATOTOX Score | p | |
|---|---|---|---|---|
| Low (n=56) | High (n=47) | |||
| Severe* thrombocytopenia (Platelet Count < 50 G/L) | ||||
| Day 0–30 | 59 (57%) | 25 (45%) | 34 (72%) | 0.006 |
| Day 31–100 | 36 (35%) | 8 (14%) | 28 (60%) | <0.0001 |
| Severe* anemia (Hb < 8 g/dL or requiring transfusion) | ||||
| Day 0–30 | 53 (51%) | 22 (39%) | 31 (66%) | 0.01 |
| Day 31–100 | 27 (26%) | 6 (11%) | 21 (45%) | 0.0001 |
| Neutropenia | ||||
|
Phenotype of Neutrophil Recovery Quick Intermittent Aplastic |
42 (41%) 39 (38%) 22 (21%) |
32 (57%) 24 (43%) 0 (0%) |
10 (21%) 15 (32%) 22 (47%) |
<0.0001 |
|
Severe (ANC <500/μL) Day 0 – 30 Day 31 – 100 |
91 (88%) 28 (27%) |
44 (79%) 5 (9%) |
47 (100%) 23 (49%) |
0.0004 <0.0001 |
|
Protracted, severe
(ANC <500/μL for ≥7 days) |
61 (59%) | 23 (41%) | 38 (81%) | <0.0001 |
|
Profound (ANC <100/μL) Day 0–100 |
66 (64%) | 26 (46%) | 40 (85%) | <0.0001 |
|
Protracted, profound (ANC <100/μL for ≥7 days) |
27 (26%) | 7 (13%) | 20 (43%) | 0.0007 |
|
Prolonged (ANC <1000/μL measured ≥21 days after CAR-T) |
48 (47%) | 17 (30%) | 31 (66%) | 0.0004 |
| Supportive therapies – n (%) | ||||
| Platelet transfusion D1–30 D30–100 |
35 (34%) 21 (20%) |
9 (16%) 2 (4%) |
26 (55%) 19 (40%) |
<0.0001 <0.0001 |
| pRBC transfusion D1–30 D30–100 |
42 (41%) 24 (23%) |
17 (30%) 5 (9%) |
25 (53%) 19 (40%) |
0.027 0.0003 |
| Granulocyte colony stimulating factor (G-CSF) use | 58 (56%) | 25 (45%) | 33 (70%) | 0.01 |
| First day of G-CSF – median (range) | 14 (0–287) | 16 (0–90) | 0 (0–287) | 0.89 |
| Last day of G-CSF – median (range) | 30 (0–365) | 28 (0–350) | 43 (0–365) | 0.17 |
| Thrombopoetin (TPO) agonist use | 8 (8%) | 2 (4%) | 6 (13%) | 0.14 |
| CD34+ Stem cell boost | 1 (1%) | N/A | 1 (2%) | 0.46 |
| Day of boost (range) | 59 | N/A | 59 | |
| Dose of boost (CD34+ cells x 106/kg) – median (range) | 2.2 | N/A | 2.2 | |
| IVIG use | 35 (34%) | 16 (29%) | 19 (40%) | 0.22 |
Severe (grade 3 or higher) according to Common Terminology of Adverse Events (CTCAE) criteria.
The CAR-HEMATOTOX score identifies patients at risk for severe hematotoxicity and transfusion dependency
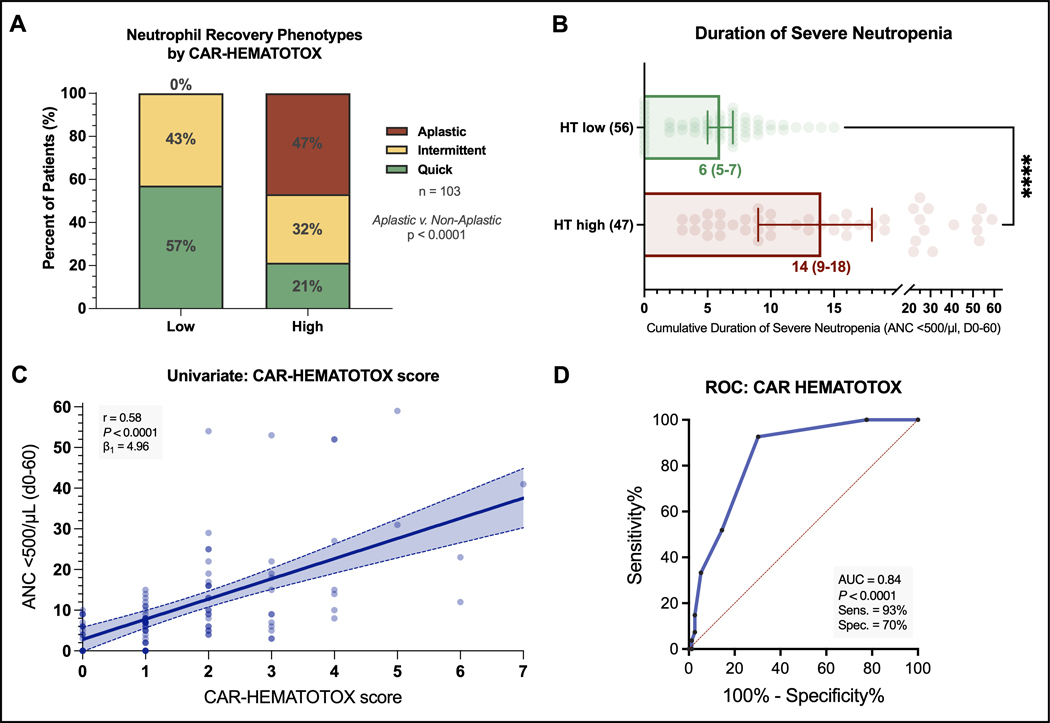
When comparing the HT score risk groups, we found that HThigh patients more frequently displayed an aplastic phenotype of neutrophil recovery, while this phenotype was not observed in the HTlow group (47% vs. 0%, p<0.0001, Figure 1A). Moreover, the HT score was an independent risk factor for the aplastic phenotype of neutrophil recovery on multivariable binary logistic regression analysis (adjusted odds ratio [aOR] = 33, 95% CI 4–270; Table S2). Serum LDH level at lymphodepletion was also independently associated with the aplastic phenotype (aOR 3.5, 95% CI 1.0–12.3). On the other hand, CRS or ICANS grade, age, use of bridging therapy, prior ASCT, and bendamustine use in the last 6 months prior to CAR-T infusion were not associated with the aplastic phenotype (Table S2). The median duration of severe neutropenia was significantly longer in HThigh patients (14 vs. 6 days, p<0.0001, Fig 1B). Consistent with these findings, we observed a significant positive correlation between the HT score and the duration of severe neutropenia on univariate analysis (r = +0.58, p<0.0001, β1=4.96; Fig. 1C). On ROC analysis, we confirmed the discriminatory capacity of the HT score in regards to the previously validated endpoint of severe neutropenia ≥14 days (AUC=0.84, p<0.0001, sensitivity=93%, specificity=70%; Fig. 1D).12
Fig. 1. The CAR-HEMATOTOX score identifies patients at risk for severe hematotoxicity prior to brexu-cel infusion.
A Relative distribution of the clinical phenotypes of neutrophil recovery (Rejeski et al Blood 2021) by HT score (red: aplastic, yellow: intermittent, green: quick). B Median total cumulative duration of severe neutropenia (ANC < 500/μL) between day 0 until day +60 by HT score. Whiskers indicate the 95% CIs, P-value determined by Mann-Whitney U test (**** p<0.0001). C Univariate analysis comparing HT score and duration of severe neutropenia (day 0–60). The Spearman correlation coefficient and respective p-value is provided. The calculated slope (β1) of the simple linear regression curve is shown, indicating an average increase in the duration of severe neutropenia of 4.96 days for every increase of 1 in the score. D Receiver operating characteristic (ROC) curve studying the HT score versus the binary outcome of severe neutropenia ≥14 days (day 0–60) vs. 0–13 days. The AUC, p-value, and test characteristics (sensitivity, specificity) are provided.
We observed higher incidence rates of hematotoxicity in the HThigh group across all cytopenia categories (Table 1). For example, protracted severe neutropenia was found in 81% HThigh patients compared to 41% in HTlow patients (p<0.0001). Protracted profound neutropenia was significantly more frequent in the HThigh group (43% vs. 13%, p=0.0007). Furthermore, the HThigh group also had an increased proportion of patients with prolonged neutropenia (66% vs. 30%, p=0.0004). Finally, the rate of both early (until day +30) and late (day +31 to day+100) severe thrombocytopenia and anemia was higher in the HThigh group. As a result, HThigh patients had increased transfusion requirement for both platelets (55% vs. 16%, p<0.0001 and 40% vs. 4%, p<0.0001) and pRBCs (53% vs. 30%, p=0.027 and 40% vs. 9%, p=0.0003) within 30 and 100 days after brexu-cel, respectively. They also more frequently required G-CSF support (70% vs. 45%, p=0.01). A numeric trend was noted for increased use of TPO agonists in HThigh patients (13% vs. 4%). A stem cell boost was administered in one HThigh patient (CD34+ selected autologous product, day +59). This patient exhibited subsequent count recovery and was in complete remission at the 3-month follow-up.
Neither the CAR-HEMATOTOX score nor the modified EASIX score was associated with the severity of CRS or ICANS
The rate of severe (grade ≥3) CRS and ICANS was 6% and 25%, respectively – comparable to prior reports (Table S3). The majority of patients (83%) had mild to moderate CRS (grade 1 to 2), while CRS was absent in 11 patients (11%). For ICANS, mild to moderate manifestations (grade 1 to 2) were observed in 38 cases (37%), while 39 patients (38%) did not develop ICANS. In terms of toxicity management, tocilizumab and corticosteroids were used in 80% and 63% of patients, respectively. The anti-interleukin-1 receptor antagonist anakinra was applied in 9 cases. Eleven patients required management in the intensive care unit (ICU). No statistically significant difference in the severity of CRS or ICANS was observed by HT score (Figures S1A–B). We also calculated the modified EASIX score at time of lymphodepletion, which was previously linked to CRS/ICANS severity in r/r LBCL patients.31 However, the score neither discriminated for CRS (Figure S1C) nor ICANS severity (Figure S1D) in our patient cohort. The median duration of hospitalization was comparable between the HT high vs. low risk groups (both 18 days, p=0.7).
Incidence and risk factors of infection following brexu-cel
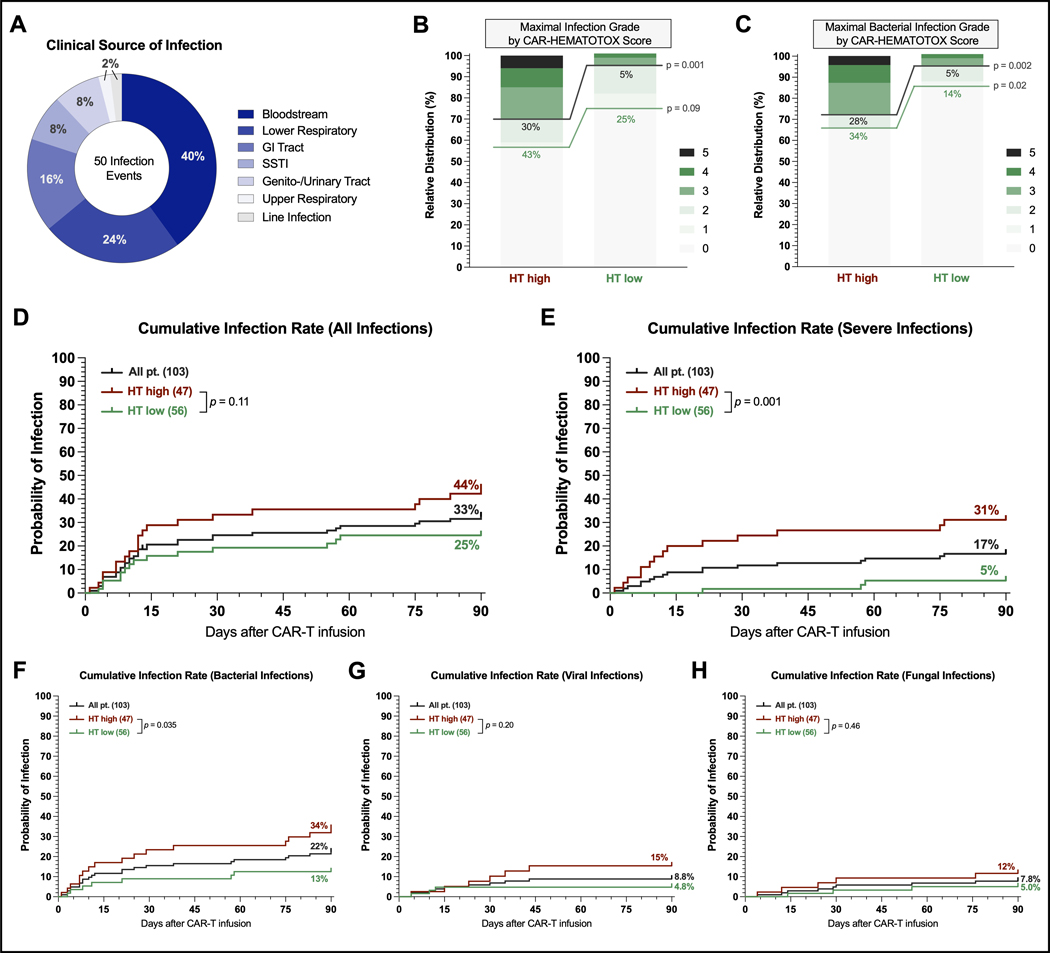
Next, we analyzed early infections occurring during the first 90 days following brexu-cel infusion. A total of 50 infection events were reported in 34 patients (33%). Bloodstream infections represented the most common source of infection (40%), followed by lower respiratory tract infections (24%) and gastrointestinal tract infections (16%) (Figure 2A). Severe infections were observed in 17 patients (15%). Risk factors on univariate analysis included HT score, age, ECOG PS, sMIPI score, aplastic phenotype, CRS/ICANS severity, and corticosteroid use (Table S4). The multivariable analysis of pre-CAR-T factors at lymphodepletion demonstrated that only the HT score was an independent risk factor for severe infections (aOR=6.5, 95% CI 1.4–31.1). Of the post-CAR-T factors, the aplastic phenotype represented the only independent adverse risk factor for severe infections (aOR 5.5, 95% CI 1.6–19.3).
Fig. 2. The CAR-HEMATOTOX score identifies patients at risk for severe infections during the first 90 days after brexu-cel infusion.
A Clinical source of infection of the 50 infection events. B Relative distribution of infection grades across all infection subtypes. Infection grades (1–5) are color-coded in shades of green with the connecting green and gray lines and percentage numbers comparing all-grade and grade ≥3 infections, respectively, in HT high versus low patients. Significance values were determined by Fisher’s exact test (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). C Relative distribution of bacterial infections by HT score. D-H Cumulative incidence rates of any-grade (D), grade ≥3 (E), bacterial (F), viral (G), and fungal (H) infections by HT score between day of CAR-T infusion (day 0) and day +90. Comparison of HT risk groups was performed by log-rank test.
The CAR-HEMATOTOX score was associated with severe infectious complications which represented the most common cause of non-relapse mortality
When studying the maximal infection grade per patient, we found that the HThigh group exhibited a higher rate of any-grade (43% vs. 25%, p=0.09) and especially severe infections (30% vs. 5%, p=0.001) compared to the HTlow group (Figure 2B). These observations were particularly evident when studying bacterial infections: both the rate of all-grade (34% vs. 14%, p=0.02) and severe infections (28% vs. 5%, p=0.002) were significantly increased in the HThigh group (Figure 2C). While no fatal infections were observed in the HTlow group during the first 90 days, we identified three deaths due to infection in the HThigh group: one fatal fungal infection on day +76 as well as two fatal bacterial infections (days +22, +40) after brexu-cel infusion. Only two patients were re-admitted for infectious episodes (1x HT high, 1x HT low), both with a hospital stay of 5 days, respectively. Importantly, the cumulative 90-day incidence of all-grade infections and especially severe infections was increased in HThigh patients (Figures 2D–E). The analysis of infection subtypes (bacterial vs. viral vs. fungal) revealed that the HT score preferentially identified patients at risk for bacterial infections (Figures 2F–H).
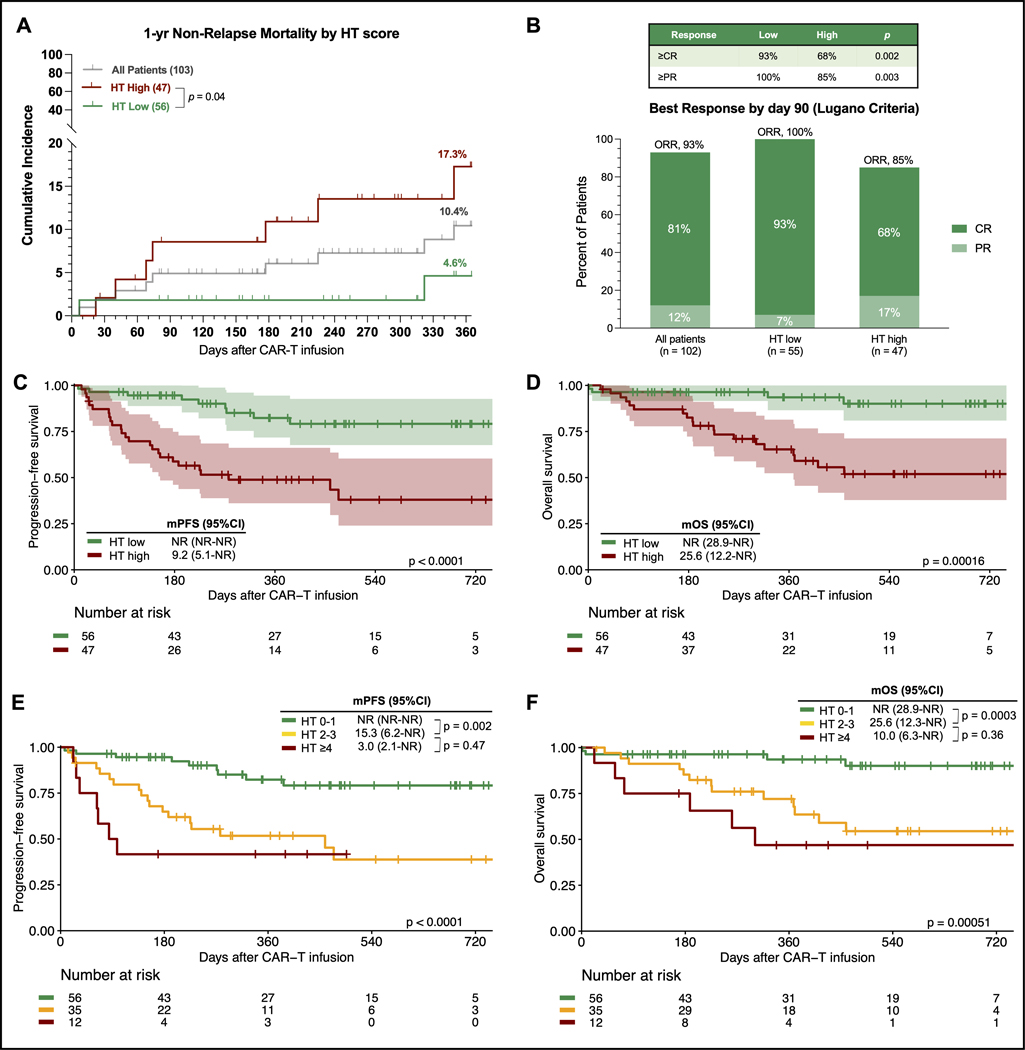
Nine patients experienced NRM by last follow-up (Figure S2): seven deaths were attributed to infection, one to severe CRS, and one to general deconditioning. The infection-related deaths were attributed to three Covid-19 infections, three bacterial infections, and one fungal infection. All three viral infections occurred in the setting of B-cell aplasia between days +177 and day +349 after brexu-cel infusion (2 HTlow, 1 HThigh patients). During the first year following CAR-T therapy, 24 patients died of lymphoma progression. The estimated 1-year NRM rate across the entire study cohort was 10.4% and was markedly higher in HThigh patients compared to their HTlow counterparts (17.3% vs. 4.6%, p=0.04, Figure 3A).
Fig. 3. The CAR-HEMATOTOX score identifies MCL patients at risk for higher non-relapse mortality and poor treatment outcomes following brexu-cel.
A 1-year NRM rate across all patients (gray) and in patients with a HT score ≥2 (high, red) vs. 0–1 (low, green) patients. The p-value of the Mantel-Cox log rank test comparing HT risk groups is depicted. B Best overall tumor response at day 90 according to Lugano criteria (Cheson et al JCO 2014) for all patients, HT low (score 0–1), and HT high (score ≥2) patients. The complete response (CR) rate is shown in dark green, partial response (PR) rate in light green. The table on the right depicts the proportion of HT low vs. high patients achieving greater than PR and/or CR, respectively. One patient did not have an evaluable response assessment due to early death. C-D Kaplan-Meier estimates of progression-free survival (PFS, C) and overall survival (OS, D) comparing HT high (red) versus low (green) patients. E-F Kaplan-Meier estimates of PFS (E) and OS (F) comparing low risk (HT score 0–1, green), intermediate to high-risk (score 2–3, yellow), and ultra high-risk patients (score ≥4, red). The superimposed tables depict the median and 95% confidence interval of survival estimates, and p-values from the univariate Cox regression analysis. The number at risk at each follow-up timepoint and the p-value of the Mantel-Cox log-rank test are provided.
Influence of the CAR-HEMATOTOX score on response to therapy and survival
The best overall response rate (ORR) by day 90 was assessed in 102 patients according to Lugano criteria. The ORR was 93%, while the complete response (CR) rate was 81% (Figure 3B). After a median follow-up of 15.4 months, median PFS was 25.2 months and median OS was not reached (Figure S3). The 1-year PFS rate was 67% (95% CI 58–77%) and 1-year OS was 80% (95% CI 72–89%). Notably, a low HT score was associated with a significantly improved ORR (100% vs. 85%, p=0.003) and superior CR rate (93% vs. 68%, p=0.002) (Figure 3B). Comparing HT risk groups, we found signicantly inferior PFS (median PFS 9.2 vs. not-reached; p<0.0001; Figure 3C) and OS in the HThigh group (median OS 25.6 months vs. not-reached; p<0.0001; Figure 3D). The HTlow patients displayed an excellent 1-year PFS and OS rate of 82% and 94%, respectively. Conversely, HThigh patients had a 1-year PFS and OS rate of 49% and 65%, respectively. We noted particularly adverse survival outcomes in patients with numerically higher HT scores of 4 or greater. These ultra high-risk candidates displayed dismal survival with a median PFS of only 3 months (95% CI 2.1 months - not reached; Figure 3E) and a median OS of 10 months (95% CI 6.3 months - not reached; Figure 3F).
Importantly, the HT score represented an independent predictor of poor PFS (adjusted hazard ratio [aHR] 3.7, 95% CI 1.7–8.0; p<0.001; Table S5) and OS (aHR 5.6, 95% CI 1.8–17.2; p=0.002; Table S6) in a multivariable Cox proportional hazards model adjusting for other pre-CAR-T factors. The sMIPI score represented another independent pre-therapy adverse risk factor for PFS (aHR 1.8, 95% CI 1.0–3.4; p=0.05), with a trend observed for OS (aHR 2.2, 95% CI 0.97–5.1, p=0.06). While serum LDH levels greater than the upper limit of normal increased the risk of poor PFS (HRPFS 2.8) and OS (HROS 2.9) on univariate analysis, statistical significance was not noted in the multivariable analysis. Studying risk factors following CAR-T administration, we found that aplastic neutrophil recovery (aHR 2.1, p=0.05) and severe infections (aHR 2.3, p=0.06) were associated with poor PFS on multivariable analysis (Table S5). Of interest, the application of corticosteroids was independently associated with improved PFS (aHR 0.4, 95% CI 0.2–0.9, p=0.02) and OS (aHR 0.3, 95% CI 0.1–0.8, p=0.015).
Discussion
In this multicenter observational study of 103 r/r MCL patients receiving brexucabtagene autoleucel, we observed a high incidence rate of hematological toxicity and severe infectious complications. We demonstrate that the CAR-HEMATOTOX score identified patients at high risk for these clinically relevant toxicities and was associated with increased therapy-related NRM. Furthermore, high HT scores were associated with an inferior response rate and poor PFS and OS.
Overall, the reported incidence of prolonged cytopenias in patients receiving brexu-cel was high in this predominantly real-world cohort. The data are consistent with results from the registrational ZUMA-2 study of 68 patients that reported grade 3 or higher cytopenias and infections in 94% and 32% of patients, respectively.5 Similarly, Iacoboni and colleagues outlined that about approximately half of all patients exhibited grade ≥3 cytopenias at the 1-month follow-up in a small real-world cohort.6 Futhermore, the depth and duration of hematological toxicity was comparable to a pooled analysis of 235 r/r LBCL patients using a similar methodology, indicating the product- and disease-independent nature of hematotoxicity.12 To ease future cross-entity comparisons, a harmonized consensus grading system has been developed for immune effector cell-associated hematotoxicity (ICAHT) by the European Hematology Association (EHA) and European Society for Blood and Marrow Transplantation (EBMT).32,33 Moreover, we confirmed that hematotoxicity in MCL patients also often manifests in the form of biphasic neutropenia with recurrent neutrophil dips (“intermittent phenotype”, 38% of patients). Interestingly, approximately half of all brexu-cel treated patients exhibited prolonged neutropenia after day +21. The occurrence long after lymphodepleting chemotherapy and resolution of clinical CRS implies a CAR-T-related mechanism, and may reflect persistence of CAR T-cells and ongoing inflammation-related myelosuppression.25,34,35 Importantly, the persistent nature of CAR-T-related cytopenias may prevent the administration of potentially efficacious post-relapse therapies and can result in exclusion from clinical trials.36,37
In terms of the management of hematotoxicity, the majority of patients received G-CSF with a median time to first use of 14 days. In the future, an earlier trigger point may be considered, especially in high-risk patients, with recent evidence demonstrating the safety of early G-CSF prophylaxis as early as day +2 after CAR T-cell infusion.38,39 TPO agonists were applied in a small fraction of patients, but may be useful for G-CSF refractory cases and thrombocytopenia in particular.40–42 While only one patient received a stem cell boost, this represents a promising strategy to ameliorate BM failure, especially considering that a fraction of MCL patients may have received autologous stem cell transplantation prior to CAR-T therapy and have additional stem cells available in storage.43–45
While we noted an association between steroid use and improved survival in our study, the impact of glucocorticoids on the efficacy of CAR-T therapy likely is contingent on a multitude of factors including cumulative dose, duration, and timing.46–48 For example, early or preemptive steroid use can reduce the total cumulative dose applied for toxicity management following CAR-T infusion.22,49 Further data is needed to elucidate the precise prognostic impact of steroids in MCL patients receiving brexu-cel. The predictive capacity of the HT score underlines the importance of pre-CAR-T bone marrow reserve and systemic inflammation in driving toxicity and response to CAR-T therapy in the context of MCL. On the one hand, baseline cytopenias (especially thrombocytopenia) can reflect underlying BM infiltration and poor disease features.7,50 On the other hand, elevated systemic inflammatory markers hint at underlying host immune dysregulation, which plays an important role both in the pathogenesis of hematological toxicity and early infections following CD19 CAR-T.25,51 Furthermore, markers such as CRP and ferritin have been closely linked to an inflammatory tumor micromilieu in r/r LBCL, including increased tumor interferon signaling, and myeloid-derived cells both in the tumor microenvironment and the circulation.52 Similarly, an increase of both myeloid cells and exhausted T-cell populations was observed in MCL patients relapsing after CAR-T in a recent study by Jiang and colleagues.53 Such inflammatory stressors may in turn modulate the subsequent CAR T-cell expansion that is required to efficiently eradicate lymphoma cells.54 Considering that an increased CAR-HEMATOTOX score may fundamentally reflect underlying disease ‘aggressiveness’ and immune dysregulation, HThigh patients may represent attractive candidates to explore anticipatory anti-inflammatory measures such as JAK inhibitors (NCT05757219)55 or concurrent BTK inhibition.22
This study has several pertinent limitations: it was retrospective, uncontrolled, and follow-up remains short. Although the inclusion of patients from multiple centers across diverse health care settings was a strength of the analysis, this comes at the price of heterogeneity in terms of toxicity management and post-CAR-T relapse care. Response assessment was not perfomed centrally by an independent review committee. Future studies should evaluate the HT score in a prospective manner. Still, these data have several clinical implications. First, the HT score may guide risk-adapted anti-infective prophylaxis including the use of fluoroquinolones and mold-active azoles.26 For example, we recently demonstrated a reduction of severe infections with antibacterial prophylaxis in HThigh but not HTlow patients.20 Due to their lower risk of infection, HTlow patients may thus represent an ideal group to investigate antibiotic stewardship programs that reduce antibiotic exposure, which appears particularly relevant considering the association between an intact gut microbiome and CAR-T efficacy.56,57 Serial assessments of serum procalcitonin [PCT] may further help to rule out infections in the context of CRS (e.g. HTlow patients with non-elevated PCT at time of first fever).58,59 Second, the HT score could help to define patients that benefit from early and/or prophylactic G-CSF use,38,39 and identify ultra high-risk patients in whom collection of autologous CD34+ stem cells should be strongly considered before CAR-T therapy.43 Finally, MCL patients with a HT score ≥4 were less likely to have a durable response to brexu-cel and represent an unmet need. Additional therapeutic strategies such as bispecific antibodies, novel CAR constructs, and novel immunomodulatory agents need to be studied in these patients.
In conclusion, the HT score enables early risk-stratification of MCL patients into a high vs. low risk for prolonged neutropenia, severe infections, and poor survival outcomes prior to brexu-cel infusion. The score will enable individualized toxicity management strategies that spare over-treatment in low-risk candidates, while mitigating the sequelae of toxicity in high-risk patients. Finally, the score could aid with patient selection and help to identify ultra high risk candidates in need of further treatment optimization.
Supplementary Material
Figure S1 Neither the CAR-HEMATOTOX score nor the modified EASIX score at lymphodepletion were associated with the severity of CRS or ICANS A-B Relative distribution of CRS grades (A) and ICANS grades (B) according to ASTCT criteria by HT score. C-D Log 2 transformed modified EASIX score (Pennisi et al Blood Advances 2021) by CRS (C) and ICANS (D) risk category. Mild defined as ASTCT grade 1–2, severe defined as ASTCT grade ≥3. P-values determined by Kruskal-Wallis test.
Figure S2 Non-relapse mortality after brexu-cel infusion is driven by severe infections Nine patients died of non-relapse mortality (NRM) related reasons during the entire study follow-up. Pie chart depicts the distribution underlying causes of death.
Figure S3 Survival outcomes in r/r MCL patients treated with brexu-cel Kaplan-Meier estimates of progression-free survival (PFS, dark grey) and overall survival (OS, light grey). Median PFS/OS and 1-year PFS/OS rate with 95% confidence intervals (Cis) are provided.
Acknowledgements
We are particularly grateful for the support of all patients and the personnel who supported this work across all the participating centers.
Grants and Funding
KR received a fellowship from the School of Oncology of the German Cancer Consortium (DKTK) and was funded by the Else Kröner Forschungskolleg (EKFK) within the Munich Clinician Scientist Program (MCSP). This work was supported by a grant within the Gilead Research Scholar Program (to KR, MS) and by a Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) research grant provided within the Sonderforschungbereich SFB-TRR 388/1 2021 – 452881907, and DFG research grant 451580403 (to MS). This work was in part supported by NCI/NIH P30CA076292. FLL is in part supported as a Clinical Scholar by the Leukemia and Lymphoma Society, The work was further supported by the Bavarian Elite Graduate Training Network (to MS), the Wilhelm-Sander Stiftung (to MS, project no. 2018.087.1), the Else-Kröner-Fresenius Stiftung (to MS), and the Bavarian Center for Cancer Research (BZKF).
Footnotes
Competing interests
K.R. Kite/Gilead: Research Funding and travel support; Novartis: Honoraria; BMS/Celgene: Consultancy, Honoraria
Y.W. Research funding (to institution): Incyte, InnoCare, LOXO Oncology, Eli Lilly, MorphoSys, Novartis, Genentech, Genmab; Advisory board (compensation to institution): Eli Lilly, LOXO Oncology, TG Therapeutics, Incyte, InnoCare, Kite, Jansen, BeiGene; Honorarium (to institution): Kite
J.M. Consulting: Pharmacyclics/Abbvie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, Beigene, Servier, Novartis, Morphosys/Incyte, Secura Bio, TG Therapeutics, MEI, Lilly/Loxo; Research funding: Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium. Honoraria: Targeted Oncology, OncView, Curio, Kyowa, Physicians’ Education Resource, and Seattle Genetics; Speaker’s bureau: Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche.
P.S. Kite/Gilead: travel support; Honoraria; BMS/Celgene: Consultancy, Honoraria; Chugai : Honoraria; Janssen : travel support; Honoraria
G.I. Consultancy and Honoraria: Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Janssen, Sandoz, Miltenyi, AstraZeneca
V.L.B. AMGEN: Honoraria; Celgene: Research Funding; Pfizer: Honoraria; Kite/Gilead: Research Funding, Honoraria; Novaritis: Honoraria. Consultancy/Advisory, BMS: Consultancy/ Advisory, Takeda: Consultancy/Advisory.
M.D. Research Support (institution): Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche; Scientific Advisory Board: Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche; Speakers Honoraria: Astra Zeneca, Beigene, Gilead/Kite, Janssen, Lilly, Novartis, Roche
F.L.L. has a scientific advisory role with A2, Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group (GLG), Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, Umoja; and receives research support from Kite, a Gilead Company, Novartis, BMS, 2SeventyBio, and Allogene; and reports that his institution holds unlicensed patents in his name in the field of cellular immunotherapy.
G.H. reports grants and personal fees from AbbVie, BMS-Celgene, Incyte, Janssen, Kite/Gilead, MorphoSys AG, and Roche; personal fees from ADC-Therapeutics, AstraZeneca, Genmab, Takeda, and Novartis; and grants from Pfizer
P.B. declared having received honoraria from Allogene, Amgen, BMS/CELGENE, Janssen, Kite/Gilead, Incyte, Jazz Pharmaceuticals, Miltenyi Biomedicine, Novartis and Nektar.
E.B. Consultancy/Honoraria: Novartis, Kite/Gilead, Roche, Takeda and Incyte; Research funding (paid to institution) from Amgen; and travel and personal feed from Roche and Incyte.
Y.L. Research funding: Kite/Gilead, BMS, Janssen, Merck, Takeda, 2Seventy Bio. consultancy/advisory: Novartis, BMS, Janssen, Gamida Cells, NexImmune, NekTar Biotherapeutics, Pfizer, Kite/Gilead. DSMB: Pfizer, Sorrento. All funds to institution, no personal compensation.
M.S. has served on advisory board for Amgen Inc., BMS/Celgene, Gilead Sciences, Janssen, Novartis, Pfizer, and Seattle Genetics; served on the speaker’s bureau for Amgen Inc., BMS/Celgene, Gilead Sciences, Novartis, Pfizer, and Takeda; received travel, accommodations, and expenses from Amgen Inc., BMS/Celgene, and Gilead Sciences; and received research support from Amgen Inc., BMS/Celgene, Gilead Sciences, Miltenyi Biotec, MorphoSys, Novartis, Roche, and Seattle Genetics.
M.D.J. Kite/Gilead: Consultancy/Advisory and Research Funding, Incyte: Research Funding, Myeloid Therapeutics: Consultancy/Advisory.
The remaining authors have nothing to declare. None of the mentioned conflicts of interest were related to financing of the content of this manuscript.
Availability of data and material
For original data and material, please contact kai.rejeski@med.uni-muenchen.de
References
- 1.Silkenstedt E, Linton K, Dreyling M. Mantle cell lymphoma - advances in molecular biology, prognostication and treatment approaches. Br J Haematol. 2021;195(2):162–173. [DOI] [PubMed] [Google Scholar]
- 2.Hill HA, Qi X, Jain P, et al. Genetic mutations and features of mantle cell lymphoma: a systematic review and meta-analysis. Blood Adv. 2020;4(13):2927–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus Bendamustine and Rituximab in Untreated Mantle-Cell Lymphoma. N Engl J Med. 2022;386(26):2482–2494. [DOI] [PubMed] [Google Scholar]
- 4.Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and Safety of Ibrutinib Combined with Standard First-Line Treatment or As Substitute for Autologous Stem Cell Transplantation in Younger Patients with Mantle Cell Lymphoma: Results from the Randomized Triangle Trial By the European MCL Network. Blood. 2022;140(Supplement 1):1–3.35797018 [Google Scholar]
- 5.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacoboni G, Rejeski K, Villacampa G, et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Munoz J, Goy A, et al. Three-Year Follow-Up of KTE-X19 in Patients With Relapsed/Refractory Mantle Cell Lymphoma, Including High-Risk Subgroups, in the ZUMA-2 Study. J Clin Oncol. 2022:JCO2102370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke F, Hu ZH, Gerson J, et al. P1454: Real-World Outcomes of Brexucabtagene Autoleucel (Brexu-Cel) for the Treatment of Relapsed or Refractory (R/R) Mantle Cell Lymphoma (Mcl) in the United States (Us). Hemasphere. 2022. Jun 23;6(Suppl ):1336–1337. doi: 10.1097/01.HS9.0000848672.43673.8a. eCollection 2022 Jun. [DOI] [Google Scholar]
- 9.Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. [DOI] [PubMed] [Google Scholar]
- 11.Rejeski K, Jain MD, Smith EL. Mechanisms of Resistance and Treatment of Relapse after CAR T-cell Therapy for Large B-cell Lymphoma and Multiple Myeloma. Transplant Cell Ther. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rejeski K, Perez Perez A, Sesques P, et al. CAR-HEMATOTOX: A model for CAR T-cell related hematological toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logue JM, Peres LC, Hashmi H, et al. Early cytopenias and infections after standard of care idecabtagene vicleucelin relapsed or refractory multiple myeloma. Blood Adv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rejeski K, Kunz WG, Rudelius M, et al. Severe Candida glabrata pancolitis and fatal Aspergillus fumigatus pulmonary infection in the setting of bone marrow aplasia after CD19-directed CAR T-cell therapy - a case report. BMC Infect Dis. 2021;21(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rejeski K, Perez A, Iacoboni G, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 2022;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bethge WA, Martus P, Schmitt M, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022. [DOI] [PubMed] [Google Scholar]
- 22.Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141(20):2430–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. [DOI] [PubMed] [Google Scholar]
- 24.Juluri KR, Wu V, Voutsinas JM, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rejeski K, Perez Perez A, Iacoboni G, et al. Biphasic Neutrophil Recovery after CD19 CART in R/R LBCL Is Associated with Superior PFS/OS, Robust CAR T-Cell Expansion in Relation to Baseline Tumor Volume, and a Decrease of Systemic Inflammation over Time. Blood. 2022;140(Supplement 1):4549–4551. [Google Scholar]
- 26.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018;36(30):3043–3054. [DOI] [PubMed] [Google Scholar]
- 27.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dos Santos DMC, Rejeski K, Winkelmann M, et al. Increased visceral fat distribution and body composition impact cytokine release syndrome onset and severity after CD19 CAR-T in advanced B-cell malignancies. Haematologica. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JH, Logan BR, Wu J, et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant. 2016;22(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennisi M, Sanchez-Escamilla M, Flynn JR, et al. Modified-EASIX predicts severe cytokine release syndrome and neurotoxicity after Chimeric Antigen Receptor (CAR) T cells. Blood Adv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejeski K, Greco R, Onida F, et al. An International Survey on Grading, Diagnosis, and Management of Immune Effector Cell-Associated Hematotoxicity (ICAHT) Following CAR T-cell Therapy on Behalf of the EBMT and EHA. Hemasphere. 2023;7(5):e889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rejeski K, Subklewe M, Aljurf M, et al. Immune Effector Cell-Associated Hematotoxicity (ICAHT): EHA/EBMT Consensus Grading and Best Practice Recommendations. Blood. 2023. [DOI] [PubMed] [Google Scholar]
- 34.de Bruin AM, Demirel O, Hooibrink B, Brandts CH, Nolte MA. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. [DOI] [PubMed] [Google Scholar]
- 35.Tie R, Li H, Cai S, et al. Interleukin-6 signaling regulates hematopoietic stem cell emergence. Exp Mol Med. 2019;51(10):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain P, Nastoupil L, Westin J, et al. Outcomes and management of patients with mantle cell lymphoma after progression on brexucabtagene autoleucel therapy. Br J Haematol. 2021;192(2):e38–e42. [DOI] [PubMed] [Google Scholar]
- 37.Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol. 2021;39(18):1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lievin R, Di Blasi R, Morin F, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller KC, Johnson PC, Abramson JS, et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 2022;12(10):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyar-Katz O, Perry C, On YB, et al. Thrombopoietin receptor agonist for treating bone marrow aplasia following anti-CD19 CAR-T cells-single-center experience. Ann Hematol. 2022;101(8):1769–1776. [DOI] [PubMed] [Google Scholar]
- 41.Baur R, Jitschin R, Kharboutli S, et al. Thrombopoietin receptor agonists for acquired thrombocytopenia following anti-CD19 CAR-T-cell therapy: a case report. J Immunother Cancer. 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rejeski K, Wu Z, Blumenberg V, et al. Oligoclonal T-cell expansion in a patient with bone marrow failure after CD19 CAR-T for Richter transformed DLBCL. Blood. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rejeski K, Burchert A, Iacoboni G, et al. Safety and feasibility of stem cell boost as a salvage therapy for severe hematotoxicity after CD19 CAR T-cell therapy. Blood Adv. 2022;6(16):4719–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565–575. [DOI] [PubMed] [Google Scholar]
- 45.Hermine O, Jiang L, Walewski J, et al. High-Dose Cytarabine and Autologous Stem-Cell Transplantation in Mantle Cell Lymphoma: Long-Term Follow-Up of the Randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41(3):479–484. [DOI] [PubMed] [Google Scholar]
- 46.Sun Z, Xun R, Liu M, Wu X, Qu H. The Association Between Glucocorticoid Administration and the Risk of Impaired Efficacy of Axicabtagene Ciloleucel Treatment: A Systematic Review. Front Immunol. 2021;12:646450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strati P, Ahmed S, Furqan F, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Deng B, Yin Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oluwole OO, Bouabdallah K, Munoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DAS CK, Kumar L, Gogia A, Kaushal R. Bone marrow involvement in mantle cell lymphoma: An immunophenotyping analysis with clinicopathologic correlation from a tertiary care center in North India. Journal of Clinical Oncology. 2016;34(15_suppl):e19073-e19073. [Google Scholar]
- 51.Rejeski K, Blumenberg V, Forsberg SK, et al. Distinguishing Early Infections from CRS with Routine and Exploratory Serum Proteomics and the HT10 Score Following CD19 CAR-T for Relapsed/Refractory B-NHL. Blood. 2022;140(Supplement 1):7421–7422. [Google Scholar]
- 52.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137(19):2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang VC, Hao D, Jain P, et al. TIGIT is the central player in T-cell suppression associated with CAR T-cell relapse in mantle cell lymphoma. Mol Cancer. 2022;21(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pratta M, Burke L, DiPersio JF, et al. JAK1 Inhibition during CAR T-Cell Treatment Does Not Affect CAR T-Cell Proliferation, Persistence, or Function. Blood. 2022;140(Supplement 1):10350–10351. [Google Scholar]
- 56.Smith M, Dai A, Ghilardi G, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28(4):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein-Thoeringer CK, Saini NY, Zamir E, et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rejeski K, Blumenberg V, Iacoboni G, et al. Identifying Early Infections in the Setting of CRS With Routine and Exploratory Serum Proteomics and the HT10 Score Following CD19 CAR-T for Relapsed/Refractory B-NHL. Hemasphere. 2023;7(4):e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powell MZ, Mara KC, Bansal R, et al. Procalcitonin as a biomarker for predicting bacterial infection in chimeric antigen receptor T-cell therapy recipients. Cancer Med. 2023;12(8):9228–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Neither the CAR-HEMATOTOX score nor the modified EASIX score at lymphodepletion were associated with the severity of CRS or ICANS A-B Relative distribution of CRS grades (A) and ICANS grades (B) according to ASTCT criteria by HT score. C-D Log 2 transformed modified EASIX score (Pennisi et al Blood Advances 2021) by CRS (C) and ICANS (D) risk category. Mild defined as ASTCT grade 1–2, severe defined as ASTCT grade ≥3. P-values determined by Kruskal-Wallis test.
Figure S2 Non-relapse mortality after brexu-cel infusion is driven by severe infections Nine patients died of non-relapse mortality (NRM) related reasons during the entire study follow-up. Pie chart depicts the distribution underlying causes of death.
Figure S3 Survival outcomes in r/r MCL patients treated with brexu-cel Kaplan-Meier estimates of progression-free survival (PFS, dark grey) and overall survival (OS, light grey). Median PFS/OS and 1-year PFS/OS rate with 95% confidence intervals (Cis) are provided.