Abstract
Purpose:
Sleep deficits are a common nonmotor symptom of Parkinson disease (PD). People with mild PD also achieve less physical activity (PA) than healthy older adults (HOA), but the relationship between sleep and PA in PD is unclear. This study examined associations between sleep and PA in participants with PD and HOA.
Materials and Methods:
Secondary analysis of a prospective observational study. Participants wore a commercially available activity monitor for two weeks. Wilcoxon Rank-Sum tests compared nighttime sleep, wakenings after sleep onset, number of wakenings, naps, step count, and PA intensity between PD and HOA groups. Age-adjusted regression models calculated associations between nighttime sleep and PA.
Results:
Per day, participants with PD slept 75 fewer minutes (p < 0.01), took 5,792 fewer steps (p < 0.001), achieved less PA at all intensities, and had 32% more sedentary time (p < 0.001) compared to HOA. Thirty minutes more sleep was associated with 26 fewer sedentary minutes for HOA (p = 0.01) and 25 fewer sedentary minutes for the PD group (p < 0.001).
Conclusions:
Sleep and PA are reduced in mild PD compared to HOA. Both groups demonstrated similar associations between reduced sleep and increased sedentary behavior. Results may encourage providers to screen for sleep deficits when promoting PA.
Keywords: Parkinson disease, physical activity, sleep, health promotion, activity monitor, wearable monitor
Introduction
Sleep disturbance is one of the most common nonmotor symptoms reported by people with Parkinson disease (PD) [1]. A wide variety of sleep disorders, related to both disease processes as well as medication side effects, can co-occur with PD, including insomnia, rapid eye movement (REM) sleep behavior disorder, restless leg syndrome, and narcolepsy. Sleep disturbances significantly impact overall function and quality of life for people with PD [1–3]. Insomnia is prevalent in a majority of people with PD and is defined as one or more of the following: difficulty falling asleep, difficulty staying asleep, early awakening, or nonrefreshing sleep [4]. Although sleep disturbance in PD is complex, insomnia has been demonstrated to have greater negative impact on quality of life for people with PD than motor symptoms such as gait difficulties [4,5]. Sleep disturbance often occurs early in the course of PD, and some sleep disorders, such as REM sleep behavior disorder, can precede the onset of any motor manifestations of PD, often occurring years before PD diagnosis [1].
Similar to sleep deficits, decreased physical activity (PA) occurs early in the course of PD [6]. People with newly diagnosed and mild PD have been shown to be significantly less active compared to their peers, and this decline in overall physical activity may occur even in the absence of motor impairment progression [7–9]. Studies have also demonstrated that the decline in PA in people with PD is not fully explained by physical disability, suggesting that other factors, such as poor sleep, might be at play [7,9].
The importance of maximizing PA in people with PD is highlighted by evidence that exercise may have a beneficial neuromodulatory effect on PD disease progression [10–12]. There is emerging evidence that exercise may also improve sleep in people with PD, [13–16] although there are no studies examining whether sleep deficits, in turn, impact PA in people with PD. In healthy older adults (HOA), there is evidence of a bidirectional relationship where more sleep improves PA levels and vice versa [17–21]. Multiple studies have linked higher levels of PA to improved sleep in HOA and, conversely, longitudinal studies demonstrate a significant relationship between self-reported measures of sleep quality and higher levels of subsequent PA in healthy adults [17–20]. However, it remains unknown whether there is a relationship between sleep deficits and reduced PA in people with PD, as there is for HOA [11,22,23].
To address this gap, this study aimed to use a commercially available activity monitor to examine the association between sleep and PA in participants with mild PD and in HOA. We hypothesized that participants with early PD would demonstrate an association between reduced sleep and decreased PA. A relationship between sleep and PA in people with PD would highlight the importance of identifying, monitoring, and addressing sleep deficits as a component of personalized interventions to increase PA in people with PD.
Materials and methods
This study was a secondary analysis of sleep data gathered during a prospective observational study that compared PA variables for participants with PD and HOA using a commercially available activity monitor (Fitbit Charge HR) over a two-week period [6]. While not fully validated for all sleep measures, commercially available activity monitors have some clinical advantages compared to polysomnography, the gold standard for measuring sleep, including lower cost of equipment, a less invasive procedure, and ease of interpretation [24,25]. This specific device was selected because of its accuracy in measuring heart rate in healthy adults [26] and validity for measuring step count for individuals with PD [27]. Although this specific model has not been validated against polysomnography for measuring nighttime sleep in PD, other actigraphic devices have been shown to provide good estimates of total sleep time and wake after sleep onset in this population [28] and other Fitbit models have been validated against polysomnography for measuring total sleep time in HOA [29] and in individuals with insomnia [25]. All study procedures were approved by the University of Washington institutional review board and all participants provided written informed consent.
Study participants
Healthy older adults were recruited using flyers and participants with mild PD were recruited through a clinically validated state registry as well as local support groups [30]. Eligibility criteria for all participants were: (1) a score of 26 or greater on the Montreal Cognitive Assessment Scale (MoCA); (2) ability to walk a city block without an assistive device; (3) no history of surgery in the past 3 months; (4) no restrictions on daily PA; and (5) no diagnosis of a neurologic condition (other than PD for the PD group). An additional eligibility criterion for people with PD was an established diagnosis of PD by a physician.
Procedures
Participants were asked to provide a targeted demographic and clinical history, including age, sex, medical comorbidities, and duration of PD. Cognition was assessed using the MoCA, a measure of global cognition with scores of at least 26/30 indicating normal cognition [31]. Symptoms of PD were assessed using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) scale for all participants with PD. The MDS-UPDRS is a measure of global disease severity that includes self-report of non-motor and motor experiences and motor complications and a motor exam. The MDS-UPDRS motor examination section is scored from 0 to 132 with higher numbers indicating higher levels of motor impairment [32].
Participants were instructed to wear the activity monitor on their nondominant wrist for 14 days and 14 nights continuously except for the time needed to charge the device and during water-related activities. Activity monitors were connected to the Fitabase [Small Steps Labs LLC] for data monitoring and management. Using this platform, we analyzed minute-by-minute data for all participants to determine sleep variables, physical activity variables, and the percentage of time the monitor was removed during the study period. Each minute was uniquely assigned to one of six mutually exclusive categories: (1) non-wear time, (2) sleep time, (3) sedentary time, (4) light intensity activity, (5) moderate intensity activity, or (6) high intensity activity. Data were analyzed for participants who met acceptable adherence, which was defined as wearing the activity monitor for at least 11 out of 14 days and 11 out of 14 nights as well as at least 80% of the total time during the days and nights when the device was worn. Participants completed data activity logs to verify that no bouts of significant PA or sleep were present during non-wear time.
Nighttime sleep variables
The activity monitor reports all minutes as either sleep or physical activity based on a proprietary algorithm developed by the device manufacturer that combines actigraphy and heart rate measures. The primary variable for nighttime sleep duration was total minutes of nighttime sleep, defined as the number of minutes of sleep when the sleep episode began between 8:00 PM and 7:00 AM. Additional variables included number of wakenings (NWAK) and wake time after sleep onset (WASO), defined as minutes awake between sleep onset and wake time.
Daytime sleep variables
Additional sleep variables derived from actigraphy measures included total minutes of daytime sleep and nap count. Daytime sleep was defined as bouts of sleep starting between 7:01 AM and 7:59 PM that were not continuous with nighttime sleep.
Physical activity variables
PA variables used in the analysis included daily step count as well as daily minutes of PA in the following intensity categories, defined based on American Academy of Sports Medicine MET categories: (1) high intensity (≥ 6 METs); (2) moderate intensity (3–6 METs), (3) light intensity (1–3 METs), and (4) sedentary (<1 MET) [33]. Averages for all PA and sleep variables were derived based on the number of days and nights the monitor was worn.
Statistical analysis
Descriptive statistics were used to summarize participant demographic characteristics as well as sleep and PA variables for each group. Frequency distributions of all sleep and PA variables were plotted as histograms, and normality was assessed through visual inspection prior to running comparison tests. The PD and HOA groups were then compared using parametric or non-parametric tests, as appropriate. Chi-squared tests for independence were used to compare proportions of participants in both groups who met acceptable adherence. Unpaired t-tests were used to compare age between groups. Due to lack of normally distributed data, Wilcoxon rank-sum tests were used to compare number of comorbidities and all wear time, sleep, and PA variables between groups.
The associations between sleep and all PA variables were examined using individual linear regression models with total minutes of nighttime sleep as the outcome and all PA variables as predictors, adjusted for average age, for the PD and HOA subgroups. To correct for multiple comparisons, a Bonferroni adjustment was used, with all tests set at significance level alpha ≤ 0.01. All analyses were performed using R version 3.5.1, R Foundation for Statistical Computing (Vienna, Austria).
Results
Thirty participants with PD and thirty HOA met eligibility criteria and consented to participate in the study. One participant in the HOA group cited inability to sleep while wearing the activity monitor due to the presence of a small light on the wristband and was not included. Seven participants (5 with PD and 2 HOA) did not meet minimum criteria for wearing the monitor at least 11 nights of sleep monitoring and were not included in the final analysis. For included participants, the average percentage of time during the 14-day period that the device was not worn was 6% in the HOA group and 5% in the PD group (Table 1). The proportion of participants meeting 11/14 days and nights of wear and the average wear time were not statistically significantly different between groups. Three subjects who met adherence, one in the HOA group and two in the PD group, had daytime periods of multiple hours when they removed the activity monitor, but logs reflected that these periods were not during times of significant PA. For the PD group, the average total MDS-UPDRS score was 29, the average motor section score was 12, and the median Hoehn & Yahr stage was 1, indicating mild PD symptoms and minimal or no functional impairment [34]. Both groups were similar in terms of age, MoCA score, and number of comorbidities.
Table 1.
Descriptive statistics for demographics and average daily sleep and physical activity variables for participants with Parkinson Disease and Healthy Older Adults.
| Clinical characteristics | PD (n = 25) Mean (SD) | HOA (n = 27) Mean (SD) | ||
|---|---|---|---|---|
| Difference HOA-PD (95% CI) | p-Value | |||
| MDS-UPDRS Total Score | 28 (16) | NA | ||
| MDS-UPDRS Motor Examination Section Score | 12 (9) | NA | ||
| MoCA score | 29.1 (1.8) | 29.2 (0.9) | 0.1 (−0.7,1.0) | 0.4* |
| Demographics | ||||
| Age (years) | 69 (6) | 67 (5) | −2 (−5.0, 1.2) | 0.21† |
| Comorbidities (#) | 1 (1) | 1 (1) | 0 (−1.0, 0.4) | 0.45* |
| Adherence | ||||
| Participants adherent to monitoring | 25/30 (83%) | 27/30 (90%) | ‐ | 0.45‡ |
| Time monitor removed (%) | 5 (3) | 6 (4) | 1 (−1.8,1.6) | 0.88* |
| Sleep | ||||
| Nighttime Sleep | ||||
| Total Nighttime Sleep (min) | 347 (108) | 422 (41) | 75 (27, 129) | < 0.01 * |
| Number of Wakenings (#) | 1.9 (1.3) | 2.2 (1.32) | 0.3 (−0.5, 0.9) | 0.50* |
| Wake Time after Sleep Onset (min) | 5.2 (3.4) | 6.0 (4.0) | 0.8 (−1.4, 2.4) | 0.56* |
| Daytime Sleep | ||||
| Total Daytime Sleep (min) | 112 (129) | 71 (135) | −41 (−114, 33) | 0.12* |
| Number of Naps (#) | 1.3 (1.6) | 0.6 (1.2) | −0.7 (−1.5, 0.1) | 0.07* |
| Physical Activity | ||||
| Daily Quantity | ||||
| Number of steps (#) | 5953 (2365) | 11 745 (3891) | 5792 (3886, 7678) | 0.001 * |
| Daily Intensity | ||||
| Sedentary < 1 MET (min) | 846 (122) | 642 (88) | −204 (−262, −137) | 0.001 * |
| Light Intensity 1–3 METs (min) | 172 (60) | 266 (62) | 94 (58, 127) | 0.001 * |
| Moderate Intensity 3–6 METs (min) | 15 (11) | 31 (17) | 16 (7, 24) | 0.001 * |
| High Intensity ≥ 6 METS (min) | 19 (14) | 41 (26) | 22 (7, 34) | 0.01 * |
Wilcoxon Rank-Sum Tests
Un-paired T-tests
Pearson’s Chi-Squared Test.
Italicized p-values indicate a significant difference between groups at α = 0.01.
PD: Parkinson Disease; HOA: Healthy Older Adults; MDS-UPDRS: Movement Disorders Society Unified Parkinson’s Disease Rating Scale; MET: metabolic equivalent.
Participants with PD slept on average 75 fewer minutes per night, 18% less than HOA (Table 1, p < 0.01). The average NWAK and WASO were comparable between groups. People with PD had comparable minutes of daytime sleep but demonstrated a trend toward more frequent naps compared to HOA.
Participants with PD had significantly lower PA across all measures. Participants with PD achieved on average 5792 fewer steps per day, 49% fewer than HOA, (Figure 1, p < 0.001). In addition to differences in overall step count, participants with PD also achieved significantly fewer average minutes per day in high-, moderate-, and light-intensity activities and participated in <1 MET activities, classified as sedentary time, for 204 more minutes per day compared to HOA (p < 0.001).
Figure 1.
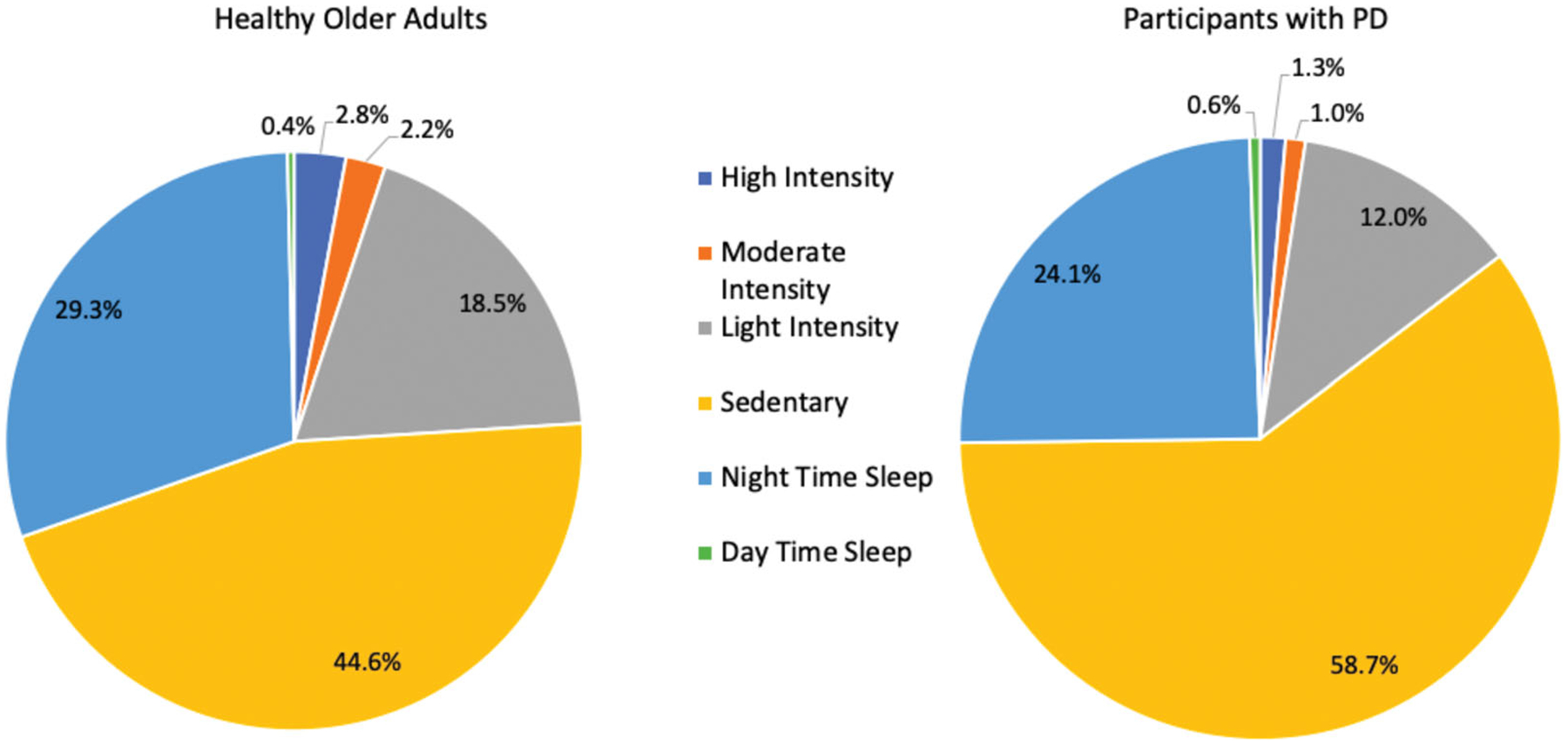
Proportion of sleep and physical activity over a 24-h period for people with Parkinson disease (PD) and healthy older adults. Numbers may not add up to 100% due to rounding.
Age-adjusted regression analysis provided no evidence of significant relationships between average nighttime sleep and average steps per day in either group. There were also no statistically significant associations between average nighttime sleep and high, moderate or light intensity active minutes for both groups (Table 2). Both HOA and participants with PD demonstrated similar statistically significant relationships between average minutes of nighttime sleep and average sedentary time, with increased sleep being associated with decreased sedentary time. Each additional 30 min of nighttime sleep was associated with 26 fewer minutes of average sedentary time per day for the HOA group (p = 0.01) and 25 fewer sedentary minutes for the PD group (Figure 2, p < 0.001).
Table 2.
Linear regression coefficients for the association between 30 min additional nighttime sleep and physical activity variables for participants with Parkinson Disease and healthy older adults.
| Dependent Variable | PD (n = 25) | HOA (n = 27) | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p-Value | Standardized Estimate/ß Weight (95% CI) | Estimate (95% CI) | p-Value | Standardized estimate/ß weight (95% CI) | |
| Steps per Day | 0.3 (−370, 371) | 1.0 | <0.01 (−10.0, 10.0) | 60 (−998, 1118) | 0.91 | <0.01 (−39.9, 39.9) |
| High Intensity Minutes | −0.3 (−2, 2) | 0.74 | −0.08 (−0.14, −0.02) | 5.3 (−0.3, 11) | 0.06 | 0.28 (0.03, 0.53) |
| Moderate Intensity Minutes | 0.6 (−1, 2) | 0.37 | 0.18 (0.14, 0.23) | 0.6 (−4, 5) | 0.78 | 0.05 (−0.13, 0.23) |
| Light Intensity Minutes | 1.6 (−5, 8) | 0.60 | 0.10 (−0.15, 0.35) | −14 (−39, 1) | 0.06 | −0.31 (−0.89, 0.27) |
| Sedentary Minutes | −25 (−37, −13) | <0.001 | −0.74 (−1.09, −0.39) | −26 (−44, −8) | 0.01 | −0.40 (−1.20, 0.40) |
All regressions adjusted for age. Italicized p-values indicate a statistically significant association at α = 0.01. PD: Parkinson Disease; HOA: Healthy Older adults.
Figure 2.
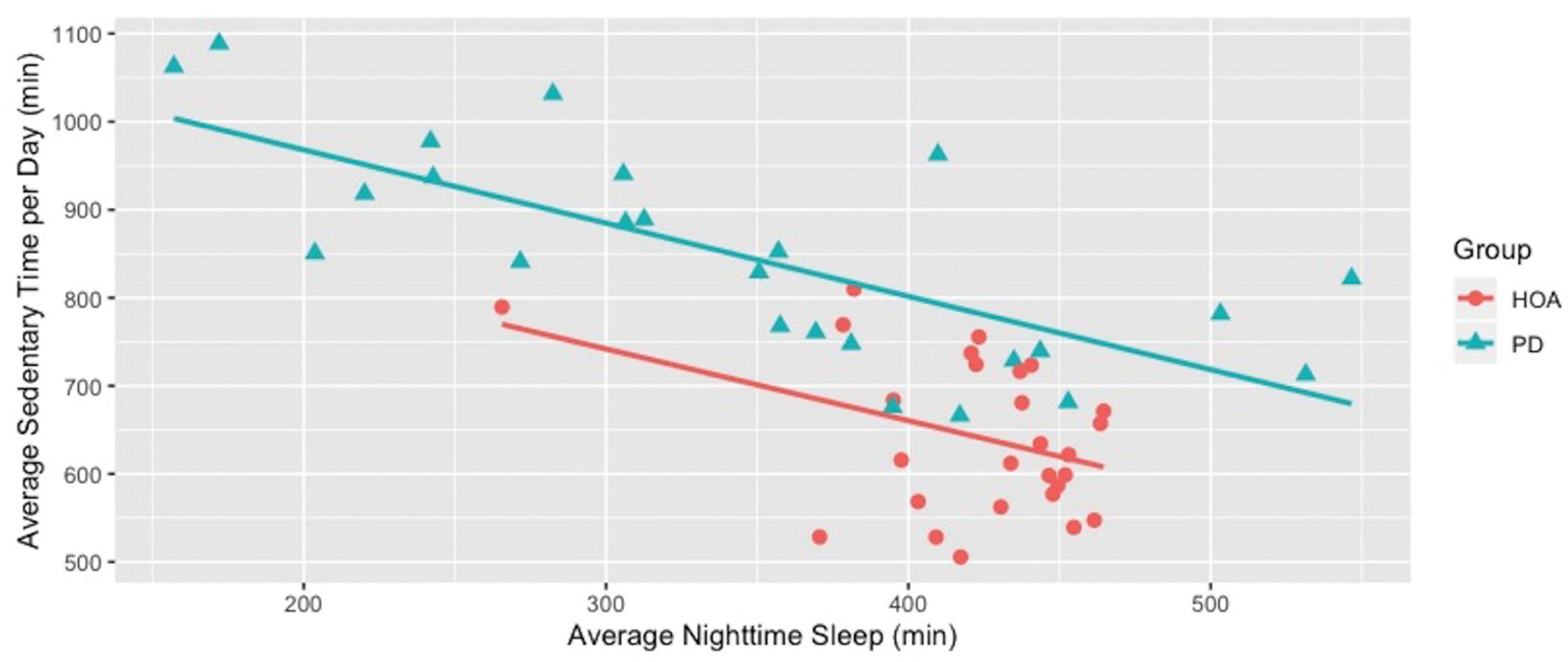
For both healthy older adults (HOA) and people with Parkinson Disease (PD), sedentary minutes decreased at similar rates with improved sleep. Each additional 30 additional minutes of nighttime sleep was associated with a reduction in sedentary minutes of 25 min in PD and 26 min in HOA.
Discussion
This study tested the hypothesis that less sleep was associated with lower PA in people with PD. Over the two-week study period, people with PD demonstrated less nighttime sleep, fewer active minutes at all intensity levels, and more sedentary minutes than HOA. The HOA group achieved an average of over 11,000 steps per day. This is unsurprising considering residents of King County, Washington consistently report high rates of PA, with Seattle ranking in the top five cities for adults completing any exercise or aerobic activities on national surveys [35,36]. Interestingly, sleep deficits were not associated with fewer overall steps but were associated with more sedentary minutes in both people with PD and HOA. This suggests that it is important to consider both the intensity and duration of PA when examining the relationship between PA and sleep, as steps alone could be spread out across the day and accumulated in short bouts.
Consistent with other studies of subjective sleep deficits in early PD, [37] the data from this study provide preliminary evidence that participants with mild PD demonstrate reduced nighttime sleep and PA early in the disease process compared to HOA of similar age and medical complexity. In this study, participants with PD got an average of 75 fewer minutes of sleep per night than HOA, similar to results from a study by Yong et al. that used polysomnography, the gold standard for measuring sleep, where participants with PD had 63 fewer minutes of nighttime sleep than age and gender-matched HOA [38]. Similar to previous work by van Nimwegen et al. demonstrating 29% lower self-reported PA for people with PD compared to matched controls, participants with PD in our sample performed 49% fewer steps per day compared to the HOA group and fewer minutes of PA across all >1 MET intensity levels than the HOA group [7].
Our data also suggest that the relationship between more sedentary behavior and less sleep may be similar in HOA and people with PD who are in the early stages of the disease. While regression analysis did not confirm previous research indicating a significant relationship between more sleep and higher quantity and intensity of PA in participants with PD or in HOA, [17,18,39] our data are consistent with other studies demonstrating that higher daytime sedentary behavior is associated with measures of poor sleep, such as reduced self-reported sleep quality and reduced sleep efficiency (minutes of sleep divided by total time in bed) in HOA [40,41].
The results of this study suggest a relationship between sleep and PA, even in early PD. Further research is needed to determine the nature of this relationship and to examine whether interventions to improve sleep can reduce sedentary behavior and improve physical activity, or vice versa, in people with PD and in HOA. Such interventions may include behavior change techniques guided by commercially available activity monitors, which have been effectively utilized to provide feedback to motivate an increase in PA in healthy populations and those with chronic illness [42–46]. Evidence for beneficial neuromodulatory effects of exercise on PD disease progression highlights the importance of any intervention that could encourage overall PA and decrease sedentary behavior in the PD population [10–12]. While the impact of sleep on motor function in PD remains unexplored, multiple studies have demonstrated that improved sleep may enhance motor learning in individuals with other neurologic diagnoses [47,48].
A recent survey demonstrated that while physical therapists agree that sleep is important for health and that poor sleep impairs function, many do not know how to assess sleep habits or sleep quality in their patients [49]. Commercially available activity monitors may have a two-fold benefit of helping providers and persons with PD monitor sleep patterns and track the relationship between sleep, exercise, and sedentary behavior to maximize function and rehabilitation outcomes [44]. Additionally, our data suggest that interventions to improve sleep may be helpful adjuncts to interventions aiming to increase PA, and vice versa, in people with mild PD, even prior to the onset of severe motor symptoms [44].
Study limitations
While clinical advantages of using commercially available monitors over more robust polysomnography measures include ease of interpretation without need for trained technicians, lower monetary and time costs, and less invasive monitoring, the commercially available activity monitor used in this study has not been specifically validated for measuring sleep in people with PD and must be interpreted with caution [24,25]. There are specific concerns about using commercially available activity monitors for measuring sleep in the PD population as the algorithms used to differentiate between sleep and awake time based on activity patterns may be impacted in populations with movement abnormalities such as decreased arm movements [29,50]. However, the use of heart rate in addition to movement patterns to differentiate between sleep and PA makes it less likely that sleep would be falsely detected by the device used in this study based on decreased arm movements alone. Additionally, some work has been done towards validating Fitbit devices and other actigraphy monitors for measuring sleep in HOA and people with PD. In HOA, FitBit devices are accurate for measuring total sleep time compared to polysomnography, but are less accurate for other sleep variables such as sleep efficiency [29]. In people with PD, actigraphy has been shown to correlate with subjective measures of sleep quality, however these results have not been replicated with commercially available sleep monitors [51]. Other research in people with PD demonstrated moderate correlations between actigraphy and polysomnography for total sleep time and WASO, but correlations between actigraphy and polysomnography declined in more advanced PD disease stages [28,50]. Further research replicating these results using more robust measures of sleep is warranted. However, the mild motor deficits of the participants with PD in this study reduces the likelihood that the activity monitor algorithm functioned differently between the PD and HOA groups. Finally, any overestimates of daytime sleep due to measurement error would be consistent within users, would strengthen our results by biasing the relationship between sedentary time and nighttime sleep towards the null, and would not preclude the use of these devices to inform individual treatment.
As a secondary data analysis, additional limitations include that the sample size and study methods and design were predetermined based on the primary research question and on the time and funding resources available. Future research could incorporate larger sample sizes or research-grade activity monitors to further elucidate the relationship between sleep and PA among people with PD. In order to further guide treatment, research with statistical methods that allow for the analysis of the temporal relationship between nighttime sleep and subsequent PA as well as PA on subsequent sleep is warranted. Additionally, this study included only participants with mild PD. Whether there is a similar relationship between sleep and sedentary behavior in individuals with more advanced PD remains to be determined.
Conclusions
This secondary analysis of sleep and PA data demonstrates an association between reduced sleep and higher sedentary behavior in participants with mild PD and HOA as measured by commercially available activity monitors. Further research with robust measures for sleep and activity and detailed characterization of participants with PD is needed to confirm the association between sleep and sedentary behavior and to investigate any mediating factors in the complex relationship between PA and sleep, such as mood, medications, or consumption of wake-promoting substances. Given the documented impacts of exercise on sleep in people with PD, future research could also examine whether mitigating sleep deficits can reduce sedentary behavior in early stages of PD. The frequency of sleep disturbance in the PD population and the association between sleep and sedentary behavior found in this study highlight the importance of screening for sleep deficits when designing interventions to maximize PA in the PD population.
IMPLICATIONS FOR REHABILITATION.
The use of a wrist-worn commercial activity and sleep monitor was well tolerated by both healthy older adults and people with mild Parkinson Disease in this study.
People with mild Parkinson Disease slept less and were less active than a group of healthy older adults.
Less sleep was associated with more sedentary behavior in both groups.
The relationship between poor sleep and sedentary behavior in mild Parkinson Disease suggests that rehabilitation interventions may be optimized by targeting both physical activity and sleep deficits.
Funding
This work was supported by The Institute of Translational Health Sciences at the University of Washington under Grant UL1TR002319. This work was supported by the National Institutes of Health under Grant NICHD/NCMRR K01HD076183.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Chahine LM, Amara AW, Videnovic A, A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Menza M, Dobkin RD, Marin H, et al. Sleep disturbances in Parkinson’s disease. Mov Disord. 2010;25(SUPPL. 1): S117–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].French IT, Muthusamy KA, A review of sleep and its disorders in patients with Parkinson’s disease in relation to various brain structures. Front Aging Neurosci. 2016;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suzuki K, Miyamoto M, Miyamoto T, et al. Sleep disturbances associated with Parkinson’s disease. Parkinsons Dis. 2011;2011:219056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Duncan GW, Khoo TK, Yarnall AJ, et al. Health-related quality of life in early Parkinson’s disease: the impact of non-motor symptoms. Mov Disord. 2014;29(2):195–202. [DOI] [PubMed] [Google Scholar]
- [6].Pradhan S, Kelly VE. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat Disord. 2019;66:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Nimwegen M, Speelman AD, Hofman-Van Rossum EJM, et al. Physical inactivity in Parkinson’s disease. Neurology. 2011;258:2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lord S, Godfrey A, Galna B, et al. Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol. 2013;260(12):2964–2972. [DOI] [PubMed] [Google Scholar]
- [9].Cavanaugh JT, Ellis TD, Earhart GM, et al. Toward understanding ambulatory activity decline in Parkinson disease. Phys Ther. 2015;95(8):1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hou L, Chen W, Liu X, et al. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front Aging Neurosci. 2017;9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Speelman AD, Van De Warrenburg BP, Van Nimwegen M, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7(9):528–534. Published online [DOI] [PubMed] [Google Scholar]
- [12].Petzinger GM, Fisher BE, McEwen S, et al. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7): 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nascimento CMC, Ayan C, Cancela JM, et al. Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatr Gerontol Int. 2014;14:259–266. [DOI] [PubMed] [Google Scholar]
- [14].Lee J, Kim Y, Lin Y, Non-pharmacological therapies for sleep disturbances in people with Parkinson’s disease: a systematic review. J Adv Nurs. 2018;74(8):1741–1751. [DOI] [PubMed] [Google Scholar]
- [15].Amara AW, Wood KH, Joop A, et al. Randomized, controlled trial of exercise on objective and subjective sleep in Parkinson’s disease. 2020;35(6):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cristini J, Weiss M, De Las Heras B, et al. The effects of exercise on sleep quality in persons with Parkinson’s disease: a systematic review with meta-analysis. Sleep Med Rev. 2021;55:101384. [DOI] [PubMed] [Google Scholar]
- [17].Tsunoda K, Kitano N, Kai Y, et al. Prospective study of physical activity and sleep in middle-aged and older adults. Am J Prev Med. 2015;48(6):662–673. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Yang B, Varrasse M, et al. Physical activity in relation to sleep among community-dwelling older adults in China. J Aging Phys Act. 2018;26(4):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holfeld B, Ruthig JC, A longitudinal examination of sleep quality and physical activity in older adults. J Appl Gerontol. 2014;33(7):791–807. [DOI] [PubMed] [Google Scholar]
- [20].Dzierzewski JM, Buman MP, Giacobbi PR Jr, et al. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Best JR, Falck RS, Landry GJ, et al. Analysis of dynamic, bidirectional associations in older adult physical activity and sleep quality. J Sleep Res. 2019;28(4):e12769. [DOI] [PubMed] [Google Scholar]
- [22].Cusso ME, Donald KJ, Khoo TK, The impact of physical activity on non-motor symptoms in Parkinson’s disease: a systematic review. Front Med. 2016;3(35):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reynolds GO, Otto MW, Ellis TD, et al. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson’s disease. Mov Disord. 2016;31(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hamill K, Jumabhoy R, Kahawage P, et al. Validity, potential clinical utility and comparison of a consumer activity tracker and a research-grade activity tracker in insomnia disorder II: outside the laboratory. J Sleep Res. 2020;29(1): 1–10. [DOI] [PubMed] [Google Scholar]
- [25].Kahawage P, Jumabhoy R, Hamill K, et al. Validity, potential clinical utility, and comparison of consumer and research-grade activity trackers in Insomnia Disorder I: in-lab validation against polysomnography. J Sleep Res. 2020;29(1): 1–11. [DOI] [PubMed] [Google Scholar]
- [26].Stahl SE, An H, Dinkel DM, et al. How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Access Sport Exerc Med. 2016;2(1):e000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lamont RM, Daniel HL, Payne CL, et al. Gait and posture accuracy of wearable physical activity trackers in people with Parkinson’s disease. Gait Posture. 2018;63:104–108. [DOI] [PubMed] [Google Scholar]
- [28].Maglione JE, Liu L, Neikrug AB, et al. Actigraphy for the assessment of sleep measures in Parkinson’s disease. Sleep. 2013;36(8):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mantua J, Gravel N, Spencer RMC, Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors. 2016;16(5):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim HM, Leverenz JB, Burdick DJ, et al. Diagnostic validation for participants in the Washington state Parkinson disease registry. Parkinsons Dis. 2018;2018:3719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4): 695–699. [DOI] [PubMed] [Google Scholar]
- [32].Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15): 2129–2170. [DOI] [PubMed] [Google Scholar]
- [33].Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- [34].Hoehn MM, Yahr MD, Parkinsonism : onset, progression, and mortality. Neurology. 1967;17(5):427–427. [DOI] [PubMed] [Google Scholar]
- [35].American College of Sports Medicine. ACSM American Fitness Index: 2019 Summary Report. 2019. [cited 2019 Dec 12]. https://www.cdc.gov/nccdphp/dnpao/data-trends-maps/index.html.
- [36].Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and Obesity. Data, Trend, and Maps. Published 2017. [cited 2019 Dec 12]. https://www.cdc.gov/nccdphp/dnpao/data-trends-maps/index.html.
- [37].Barone P, Antonini A, Colosimo C, et al. The Priamo Study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. 2009;24(11): 1641–1649. [DOI] [PubMed] [Google Scholar]
- [38].Yong MH, Fook-Chong S, Pavanni R, et al. Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One. 2011;6(7):e22511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bonardi JMT, Lima LG, Campos GO, et al. Effect of different types of exercise on sleep quality of elderly subjects. Sleep Med. 2016;25:122–129. [DOI] [PubMed] [Google Scholar]
- [40].Madden KM, Ashe MC, Lockhart C, et al. Sedentary behavior and sleep efficiency in active community-dwelling older adults. Sleep Sci. 2014;7(2):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de Castro Toledo Guimaraes LH, Bizari Coin de Carvalho L, Yanaguibashi G, et al. Physically active elderly women sleep more and better than sedentary women. Sleep Med. 2008; 9(5):488–493. [DOI] [PubMed] [Google Scholar]
- [42].Brickwood K-J, Watson G, O’Brien J, et al. Do consumer-based activity trackers increase physical activity participation? A systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7(4):e11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mercer K, Li M, Giangregorio L, et al. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR mHealth uHealth. 2016;4(2):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Siengsukon CF, Al-Dughmi M, Stevens S, Sleep health promotion: practical information for physical therapists. Phys Ther. 2017;97(8):826–836. [DOI] [PubMed] [Google Scholar]
- [45].DiFrancisco-Donoghue J, Jung MK, Stangle A, et al. Utilizing wearable technology to increase physical activity in future physicians: a randomized trial. Prev Med Reports. 2018;12:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mercer K, Giangregorio L, Schneider E, et al. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR mHealth uHealth. 2016;4(1):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Siengsukon C, Al-Dughmi M, Al-Sharman A, et al. Sleep parameters, functional status, and time post-stroke are associated with offline motor skill learning in people with chronic stroke. Front Neurol. 2015;6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dresler M, Genzel L, Kluge M, et al. Off-line memory consolidation impairments in multiple sclerosis patients receiving high-dose corticosteroid treatment mirror consolidation impairments in depression. Psychoneuroendocrinology. 2010;35(8):1194–1202. [DOI] [PubMed] [Google Scholar]
- [49].Siengsukon CF, Al-Dughmi M, Sharma NK, A survey of physical therapists’ perception and attitude about sleep. J Allied Health. 2015;44(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- [50].Goldstone A, Baker FC, de Zambotti M, Actigraphy in the digital health revolution: still asleep? Sleep. 2018;41(9):1–2. [DOI] [PubMed] [Google Scholar]
- [51].Stavitsky K, Saurman J, McNamara P, et al. Sleep in Parkinson’s disease: a comparison of actigraphy and subjective measures. Park Relat Disord. 2010;2316(4):280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


