Abstract
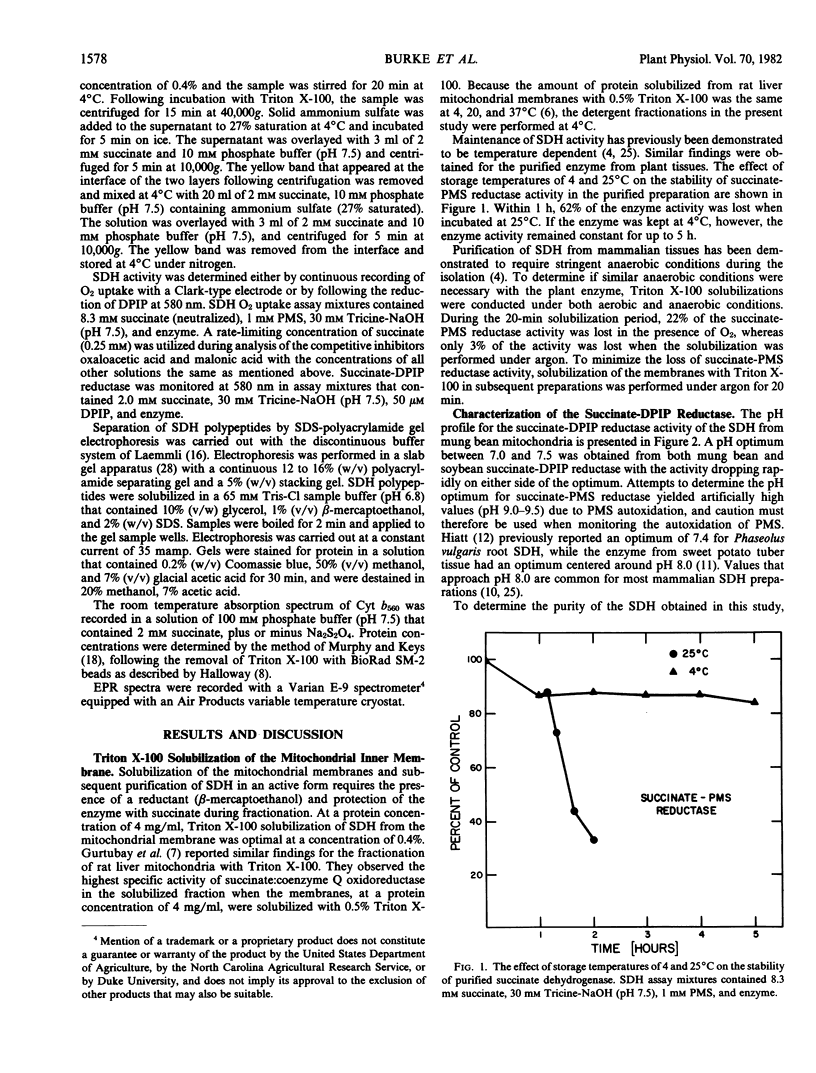
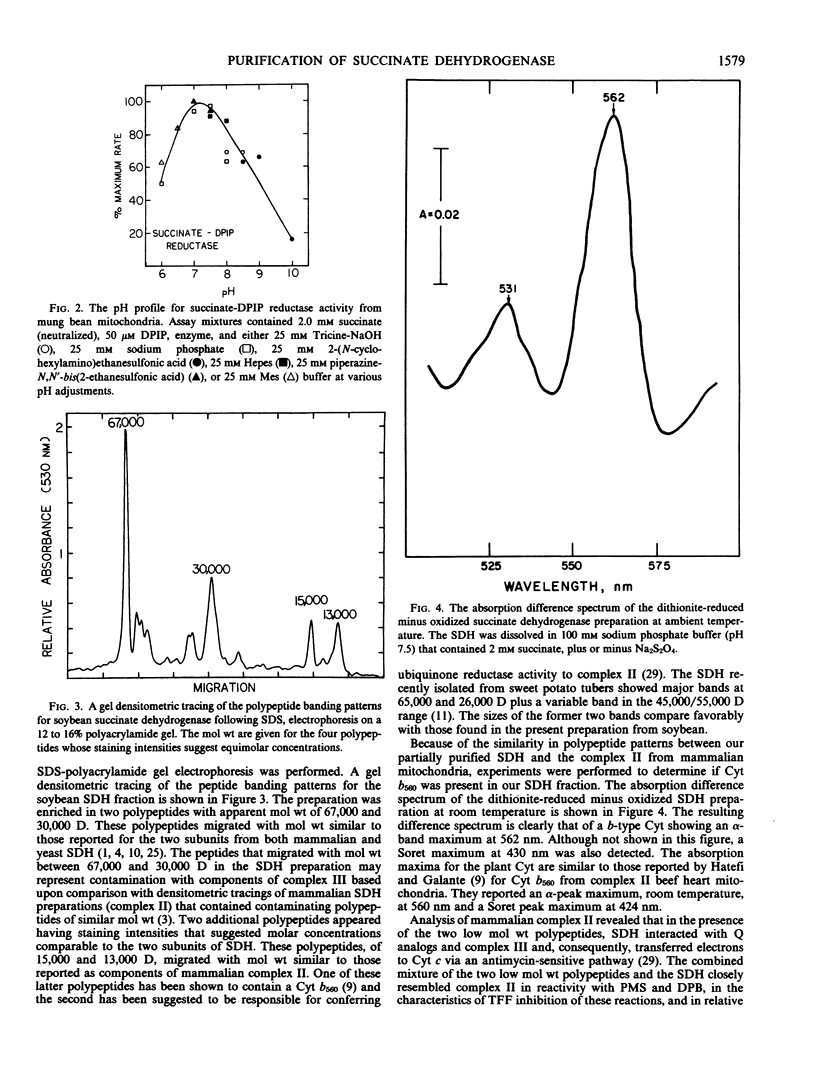
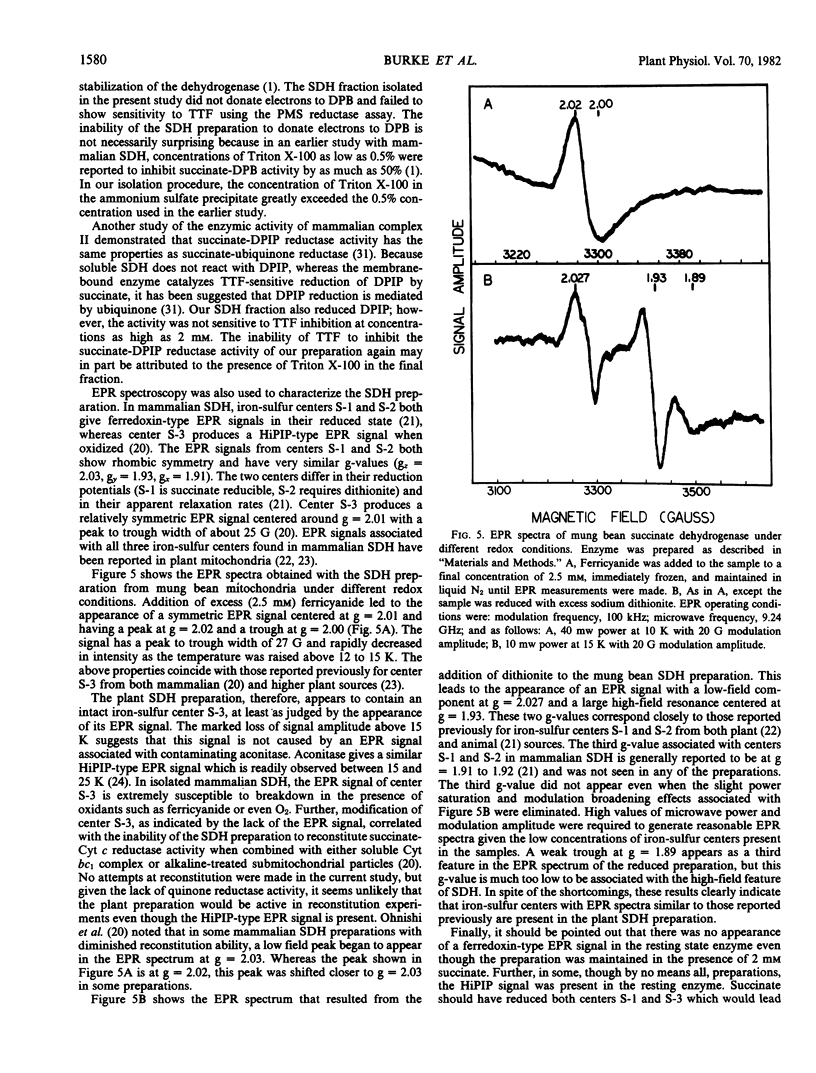
A procedure was developed for the partial purification of succinate dehydrogenase from mung bean (Vigna radiata L.) hypocotyls and soybean (Glycine max [L] Merr. v. Ransom) cotyledons. The procedure utilized a Triton X-100 extraction followed by ammonium sulfate precipitation. The final fraction was enriched in two polypeptides with approximate molecular weights of 67,000 and 30,000 daltons, exhibited a pH optima of 7.0 to 7.5, contained a b-type cytochrome, and exhibited the characteristic ferredoxin-type and high potential iron-sulfur protein-type electron paramagnetic resonance signals reported for the iron-sulfur centers of mammalian succinate dehydrogenase. Inhibition constants of 1.15 and 24.6 micromolar for oxaloacetate and malonate, respectively, were obtained.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Ball M. B., Kearney E. B. Peptides from complex II active in reconstitution of succinate-ubiquinone reductase. J Biol Chem. 1980 Apr 10;255(7):2761–2769. [PubMed] [Google Scholar]
- Ackrell B. A., Kearney E. B., Mayr M. Role 3f oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem. 1974 Apr 10;249(7):2021–2027. [PubMed] [Google Scholar]
- Capaldi R. A., Sweetland J., Merli A. Polypeptides in the succinate-coenzyme Q reductase segment of the respiratory chain. Biochemistry. 1977 Dec 27;16(26):5707–5710. doi: 10.1021/bi00645a009. [DOI] [PubMed] [Google Scholar]
- DERVARTANIAN D. V., VEEGER C. STUDIES ON SUCCINATE DEHYDROGENASE. I. SPECTRAL PROPERTIES OF THE PURIFIED ENZYME AND FORMATION OF ENZYME-COMPETITIVE INHIBITOR COMPLEXES. Biochim Biophys Acta. 1964 Nov 22;92:233–247. [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Gurtubay J. I., Goñi F. M., Gómez-Fernández J. C., Otamendi J. J., Macarulla J. M. Triton X-100 solubilization of mitochondrial inner and outer membranes. J Bioenerg Biomembr. 1980 Apr;12(1-2):47–70. doi: 10.1007/BF00745012. [DOI] [PubMed] [Google Scholar]
- Gurtubay J. I. Solubilization of inner mitochondrial membranes by triton X-100. Effect of ionic strength and temperature. Rev Esp Fisiol. 1980 Mar;36(1):83–87. [PubMed] [Google Scholar]
- Hatefi Y., Galante Y. M. Isolation of cytochrome b560 from complex II (succinateùbiquinone oxidoreductase) and its reconstitution with succinate dehydrogenase. J Biol Chem. 1980 Jun 25;255(12):5530–5537. [PubMed] [Google Scholar]
- Hiatt A. J. Preparation & some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 1961 Sep;36(5):552–557. doi: 10.1104/pp.36.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY E. B., SINGER T. P. Studies on succinic dehydrogenase. I. Preparation and assay of the soluble dehydrogenase. J Biol Chem. 1956 Apr;219(2):963–975. [PubMed] [Google Scholar]
- KEILIN D., KING T. E. Reconstitution of the succinic oxidase system from soluble succinic dehydrogenase and a particulate cytochrome system preparation. Nature. 1958 May 31;181(4622):1520–1522. doi: 10.1038/1811520a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moreland D. E., Boots M. R. Effects of optically active 1-(alpha-methylbenzyl)-3-(3,4-dichlorophenyl)urea on reactions of mitochondria and chloroplasts. Plant Physiol. 1971 Jan;47(1):53–58. doi: 10.1104/pp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Lim J., Winter D. B., King T. E. Thermodynamic and EPR characteristics of a HiPIP-type iron-sulfur center in the succinate dehydrogenase of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2105–2109. [PubMed] [Google Scholar]
- Ohnishi T., Salerno J. C. Thermodynamic and EPR characteristics of two ferredoxin-type iron-sulfur centers in the succinate-ubiquinone reductase segment of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2094–2104. [PubMed] [Google Scholar]
- Rich P. R., Bonner W. D., Jr EPR studies of higher plant mitochondria. II. Center S-3 of succinate dehydrogenase and its relation to alternative respiratory oxidations. Biochim Biophys Acta. 1978 Mar 13;501(3):381–395. doi: 10.1016/0005-2728(78)90106-8. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Solubilization, assay, and purification of succinic dehydrogenase. Biochim Biophys Acta. 1954 Sep;15(1):151–153. doi: 10.1016/0006-3002(54)90113-4. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., MASSEY V., KEARNEY E. B. Studies on succinic dehydrogenase. V. Isolation and properties of the dehydrogenase from baker's yeast. Arch Biochem Biophys. 1957 Jul;69:405–421. doi: 10.1016/0003-9861(57)90506-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L. Isolation and properties of a mitochondrial protein that converts succinate dehydrogenase into succinate-ubiquinone oxidoreductase. Biochemistry. 1980 Jul 22;19(15):3579–3585. doi: 10.1021/bi00556a025. [DOI] [PubMed] [Google Scholar]


