Abstract
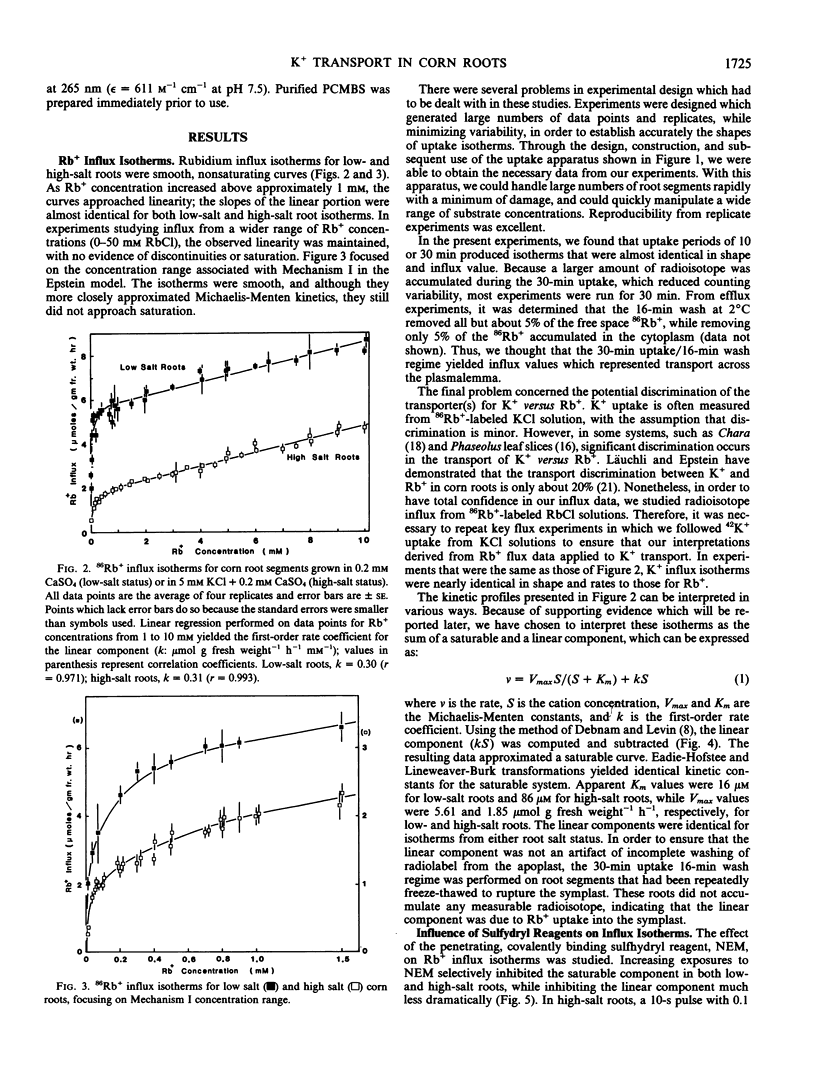
Influx isotherms were obtained for 86Rb+ uptake into 2-cm corn (Zea mays [A632 × (C3640 × Oh43)] root segments for both low- (0.2 millimolar CaSO4) and high-salt (0.2 millimolar CaSO4 + 5 millimolar KCl) grown roots. Unlike the discontinuous curves usually presented for K+ influx, our isotherms were smooth, nonsaturating curves that approached linearity at K+ (Rb+) concentrations above 1 millimolar. The kinetics for K+ transport could be resolved into saturable and linear components. The saturable components yielded Km values of 16 and 86 micromolar for low- and high-salt roots, respectively, while Vmax values were 5.62 and 1.85 moles per gram fresh weight per hour. Results of experiments with the penetrating sulfhydryl reagent, N-ethyl maleimide (NEM), and the impermeant reagent, p-chloromercuribenzene sulfonic acid (PCMBS) indicated that the saturable and linear components were independent mechanisms of K+ transport.
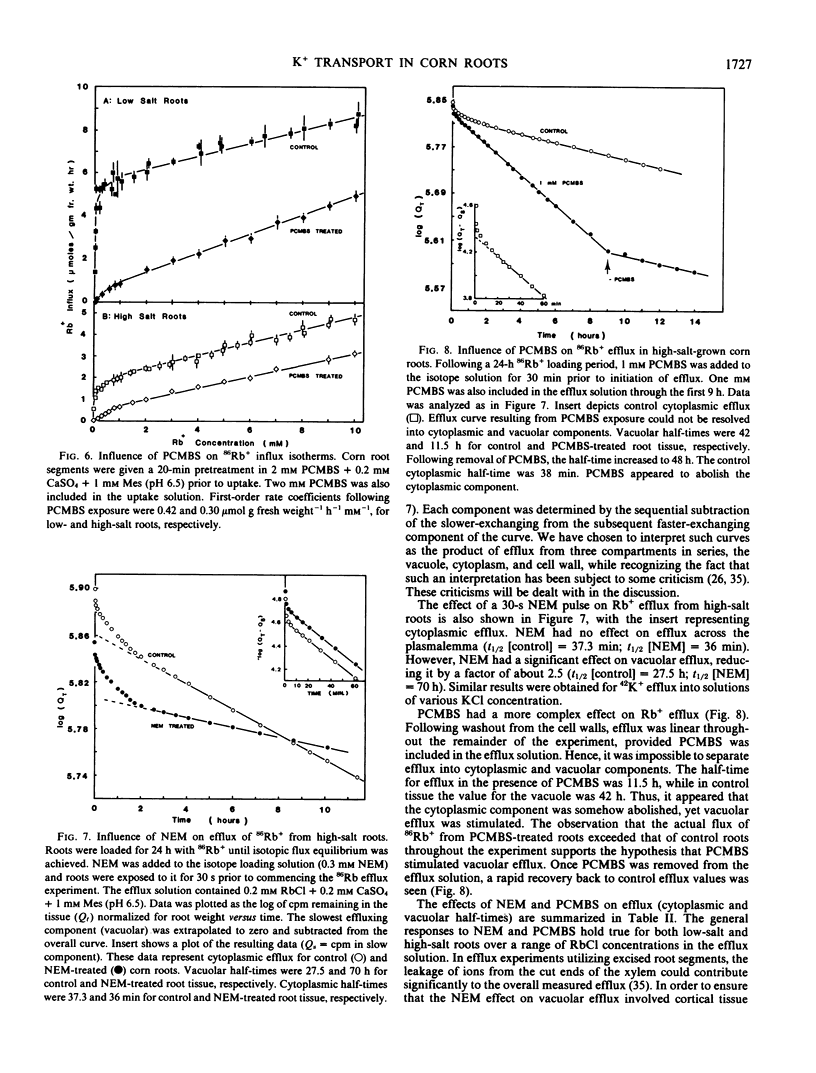
Short-term NEM exposures (30 seconds to 5 minutes) selectively inhibited the saturable system, but had little effect on the linear component. Increasing NEM exposures resulted in further inhibition and subsequent abolition of the saturable component; the linear component exhibited limited NEM sensitivity. PCMBS elicited the same general inhibitory trends, although it was less effective as a saturable component inhibitor.
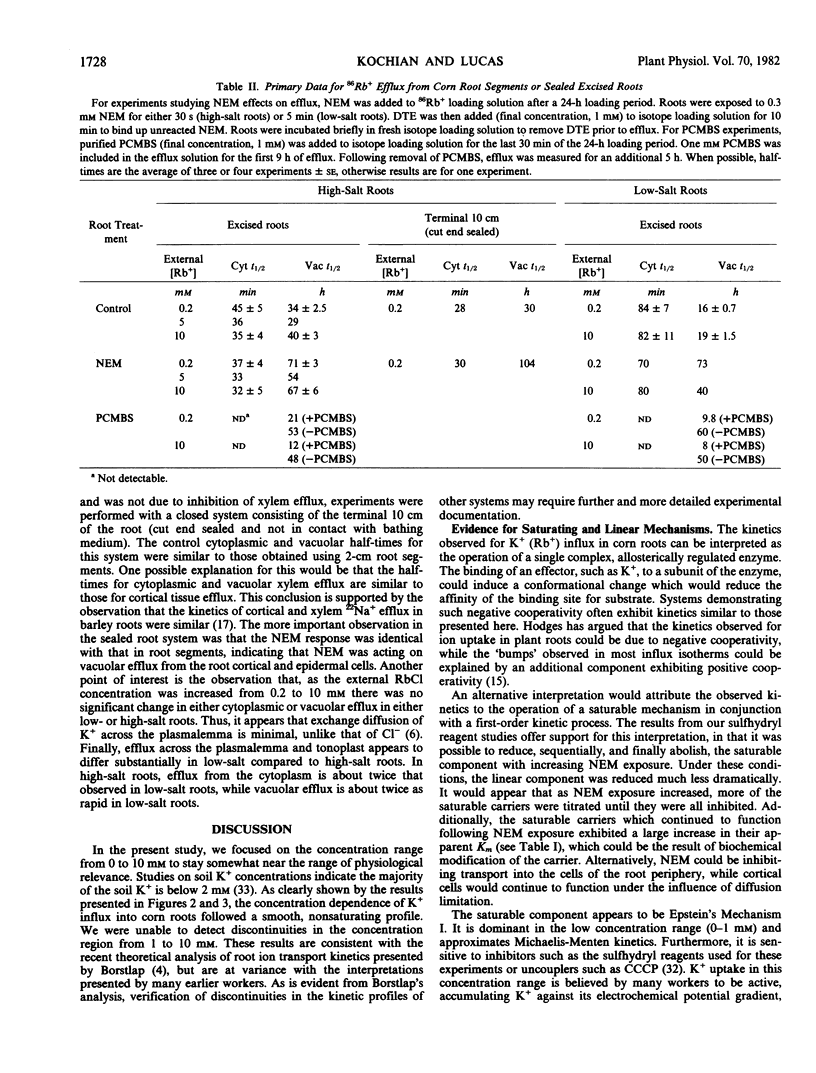
The effects of NEM and PCMBS on K+ efflux were also studied. Short NEM exposures had no effect on cytoplasmic efflux, while inhibiting vacuolar efflux significantly. From these data, it is unclear at which site(s) NEM is acting. A more complex response was obtained with PCMBS, where a monophasic efflux curve was observed. Analysis indicated that the vacuolar efflux was stimulated, while the cytoplasmic component was abolished.
The nature of the linear component is discussed, and it is proposed that the mechanism may be more complex than simple facilitated diffusion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. R. A comparison of the rate equations, kinetic parameters, and activation energies for the initial uptake of L-lysine, L-valine, gamma-aminobutyric acid, and alpha-aminoisobutyric acid by mouse brain slices. J Membr Biol. 1975 Jun 3;22(1):53–72. doi: 10.1007/BF01868163. [DOI] [PubMed] [Google Scholar]
- Cram W. J. Compartmentation and exchange of chloride in carrot root tissue. Biochim Biophys Acta. 1968 Nov 5;163(3):339–353. doi: 10.1016/0005-2736(68)90119-3. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975 Mar;246(1):181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S., Bonnemain J. L. Involvement of Protons as a Substrate for the Sucrose Carrier during Phloem Loading in Vicia faba Leaves. Plant Physiol. 1981 Mar;67(3):560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Hagen C. E. A KINETIC STUDY OF THE ABSORPTION OF ALKALI CATIONS BY BARLEY ROOTS. Plant Physiol. 1952 Jul;27(3):457–474. doi: 10.1104/pp.27.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Lüttge U. Membrane Potential Changes Related to Active Transport of Glycine in Lemna gibba G1. Plant Physiol. 1980 May;65(5):1004–1008. doi: 10.1104/pp.65.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B. Light sensitivity of na, rb, and k absorption by different tissues of bean leaves. Plant Physiol. 1975 Jun;55(6):978–981. doi: 10.1104/pp.55.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Activity of the Electrogenic Pump in Chara corallina as Inferred from Measurements of the Membrane Potential, Conductance, and Potassium Permeability. Plant Physiol. 1978 Oct;62(4):653–661. doi: 10.1104/pp.62.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Lucas W. J., Spanswick R. M. Effect of Sulfhydryl Reagents on the Biophysical Properties of the Plasmalemma of Chara corallina. Plant Physiol. 1981 Oct;68(4):899–904. doi: 10.1104/pp.68.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Sucrose uptake by developing soybean cotyledons. Plant Physiol. 1981 Sep;68(3):693–698. doi: 10.1104/pp.68.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Alexander J. M. Sulfhydryl Group Involvement in Plasmalemma Transport of HCO(3) and OH in Chara corallina. Plant Physiol. 1980 Feb;65(2):274–280. doi: 10.1104/pp.65.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A., Epstein E. Transport of potassium and rubidium in plant roots: the significance of calcium. Plant Physiol. 1970 May;45(5):639–641. doi: 10.1104/pp.45.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. A Reanalysis of the Two-Component Phloem Loading System in Beta vulgaris. Plant Physiol. 1982 Mar;69(3):734–739. doi: 10.1104/pp.69.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck B. G., Schultz S. G. Lysine transport across isolated rabbit ileum. J Gen Physiol. 1969 Feb;53(2):157–182. doi: 10.1085/jgp.53.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley L. D., Hopkins J. W. Rubidium (potassium) uptake by Arabidopsis: a comparison of uptake by cells in suspension culture and by roots of intact seedlings. Plant Physiol. 1979 Sep;64(3):374–378. doi: 10.1104/pp.64.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump B. F., Penttila A., Berezesky I. K. Studies on cell surface conformation following injury. I. Scanning and transmission electron microscopy of cell surface changes following p-chloromercuribenzene sulfonic acid (pcmbs)-induced injury of Ehrlich ascites tumor cells. Virchows Arch B Cell Pathol. 1979 Feb 6;29(4):281–296. [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]