Abstract
Epidermal and mesophyll protoplasts, prepared from leaf blades of 6-day-old light-grown Sorghum bicolor seedlings were separated by differential sedimentation and assayed for a number of enzymes. The epidermal protoplasts contained higher levels of NADPH-cytochrome c reductase (EC 1.6.2.4), triose phosphate isomerase (EC 5.3.1.1), phosphoenolpyruvate carboxylase (EC 4.1.1.31), and a UDP-glucose:cyanohydrin β-glucosyl transferase (EC 2.4.1.85), but lower levels of NADP+ triosephosphate dehydrogenase (EC 1.2.1.13) than did mesophyll protoplasts. When protoplast preparations were lysed and applied to linear sucrose density gradients, triosephosphate isomerase was found to be present in epidermal plastids. A significant fraction (41%) of the glucosyl transferase activity was also associated with the epidermal plastids.
Full text
PDF
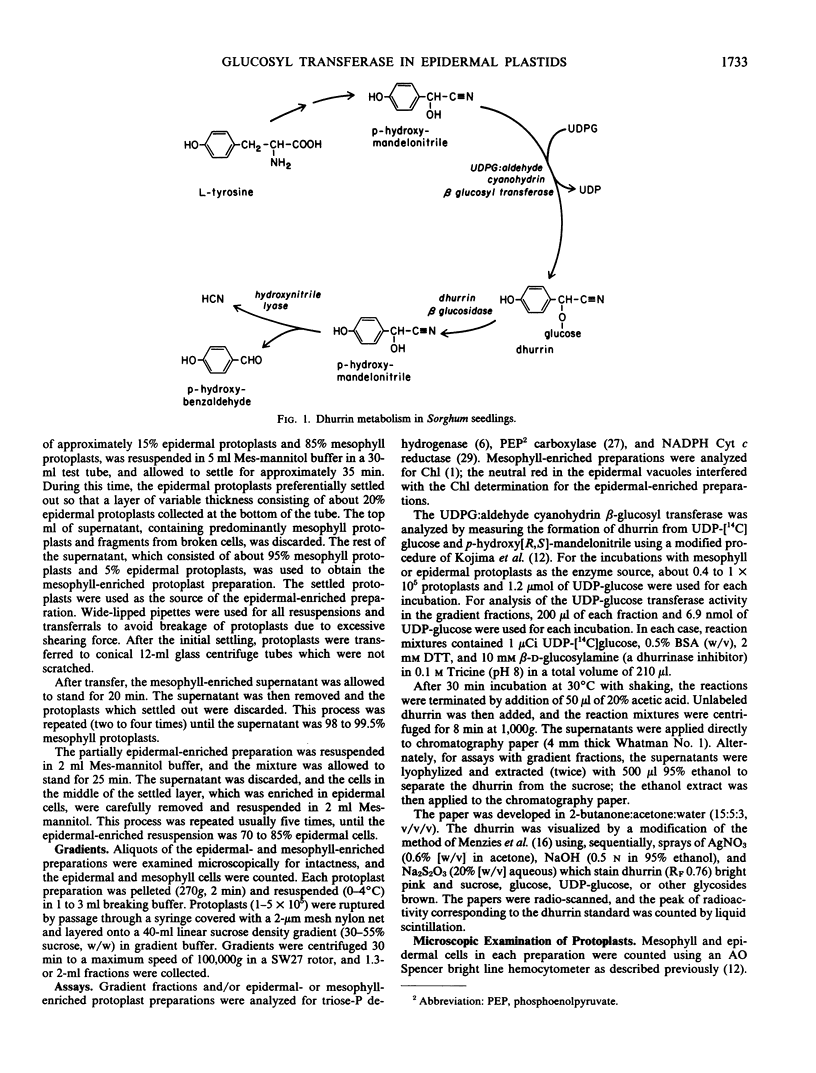
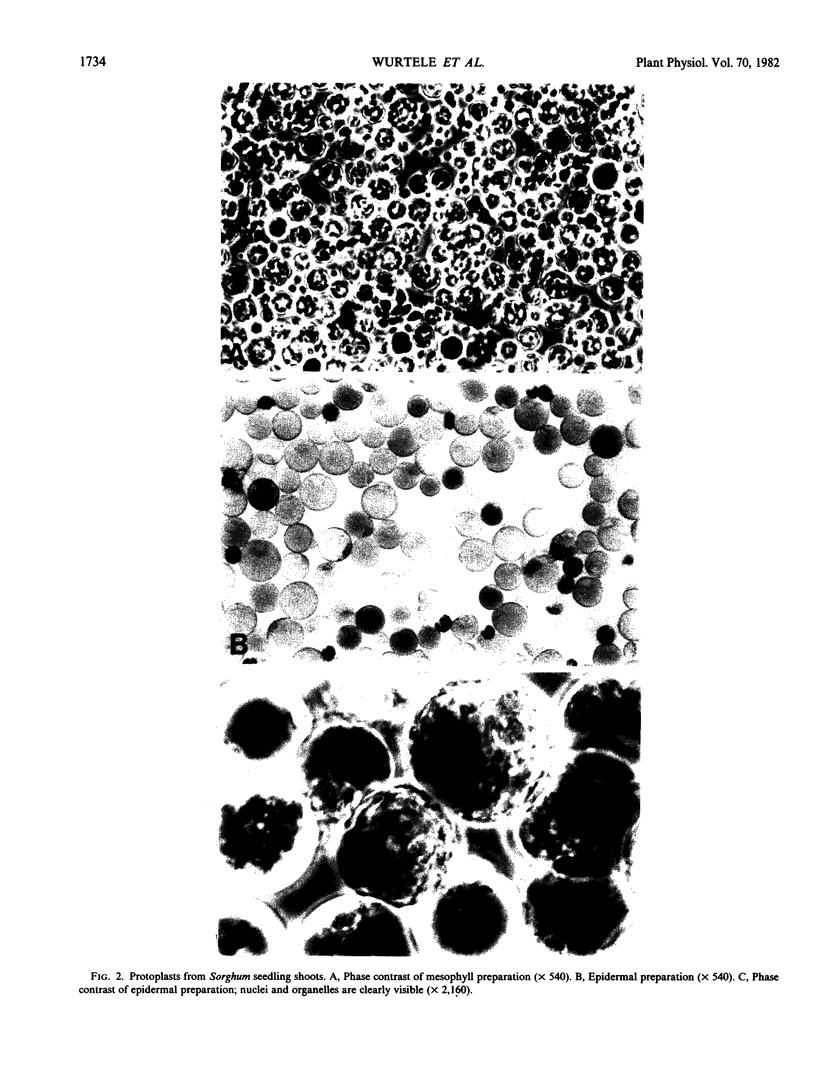


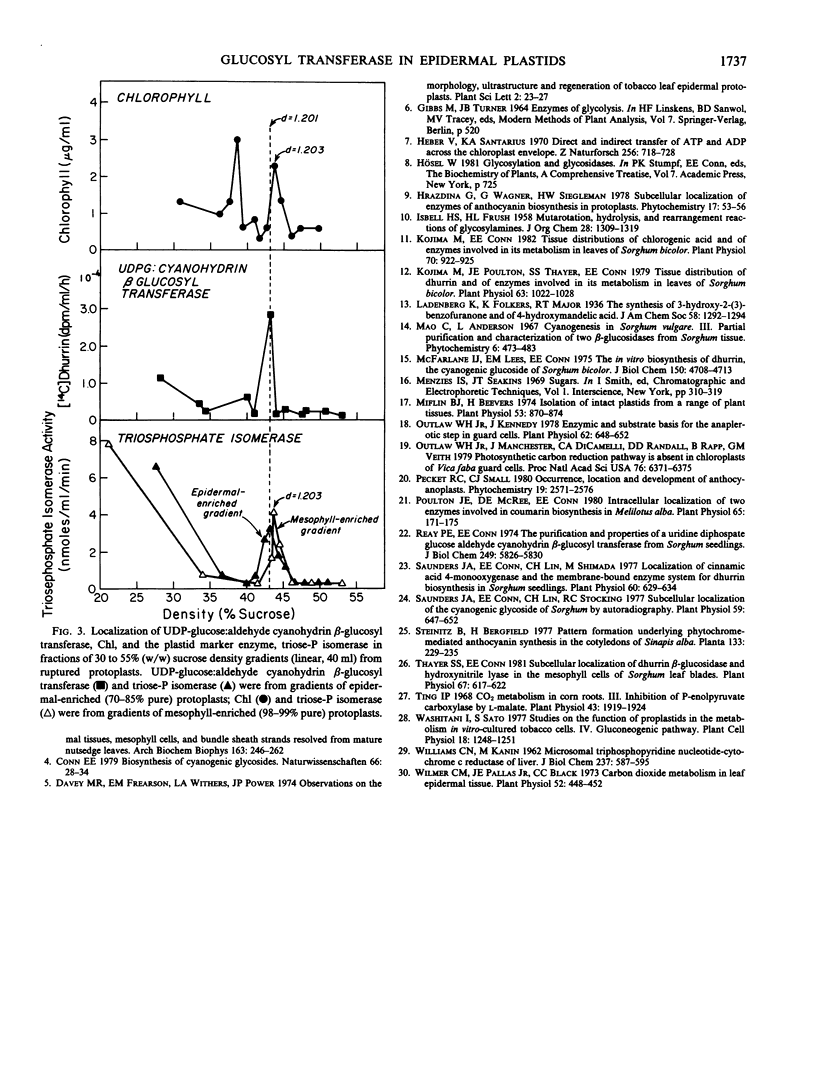
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Bruening G. The use of an abrasive in the isolation of cowpea leaf protoplasts which support the multiplication of cowpea mosaic virus. Virology. 1975 Mar;64(1):272–276. doi: 10.1016/0042-6822(75)90099-9. [DOI] [PubMed] [Google Scholar]
- Conn E. E. Biosynthesis of cyanogenic glycosides. Naturwissenschaften. 1979 Jan;66(1):28–34. doi: 10.1007/BF00369352. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Kojima M., Conn E. E. Tissue Distributions of Chlorogenic Acid and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1982 Sep;70(3):922–925. doi: 10.1104/pp.70.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane I. J., Lees E. M., Conn E. E. The in vitro biosynthesis of dhurrin, the cyanogenic glycoside of Sorghum bicolor. J Biol Chem. 1975 Jun 25;250(12):4708–4713. [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Kennedy J. Enzymic and substrate basis for the anaplerotic step in guard cells. Plant Physiol. 1978 Oct;62(4):648–652. doi: 10.1104/pp.62.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A., Randall D. D., Rapp B., Veith G. M. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton J. E., McRee D. E., Conn E. E. Intracellular Localization of Two Enzymes Involved in Coumarin Biosynthesis in Melilotus alba. Plant Physiol. 1980 Feb;65(2):171–175. doi: 10.1104/pp.65.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay P. F., Conn E. E. The purification and properties of a uridine diphosphate glucose: aldehyde cyanohydrin beta-glucosyltransferase from sorghum seedlings. J Biol Chem. 1974 Sep 25;249(18):5826–5830. [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E., Lin C. H., Shimada M. Localization of Cinnamic Acid 4-Monooxygenase and the Membrane-bound Enzyme System for Dhurrin Biosynthesis in Sorghum Seedlings. Plant Physiol. 1977 Oct;60(4):629–634. doi: 10.1104/pp.60.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Subcellular localization of the cyanogenic glucoside of sorghum by autoradiography. Plant Physiol. 1977 Apr;59(4):647–652. doi: 10.1104/pp.59.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Conn E. E. Subcellular Localization of Dhurrin beta-Glucosidase and Hydroxynitrile Lyase in the Mesophyll Cells of Sorghum Leaf Blades. Plant Physiol. 1981 Apr;67(4):617–622. doi: 10.1104/pp.67.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P. CO(2) Metabolism in Corn Roots. III. Inhibition of P-enolpyruvate Carboxylase by l-malate. Plant Physiol. 1968 Dec;43(12):1919–1924. doi: 10.1104/pp.43.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]