Abstract
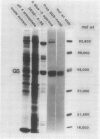
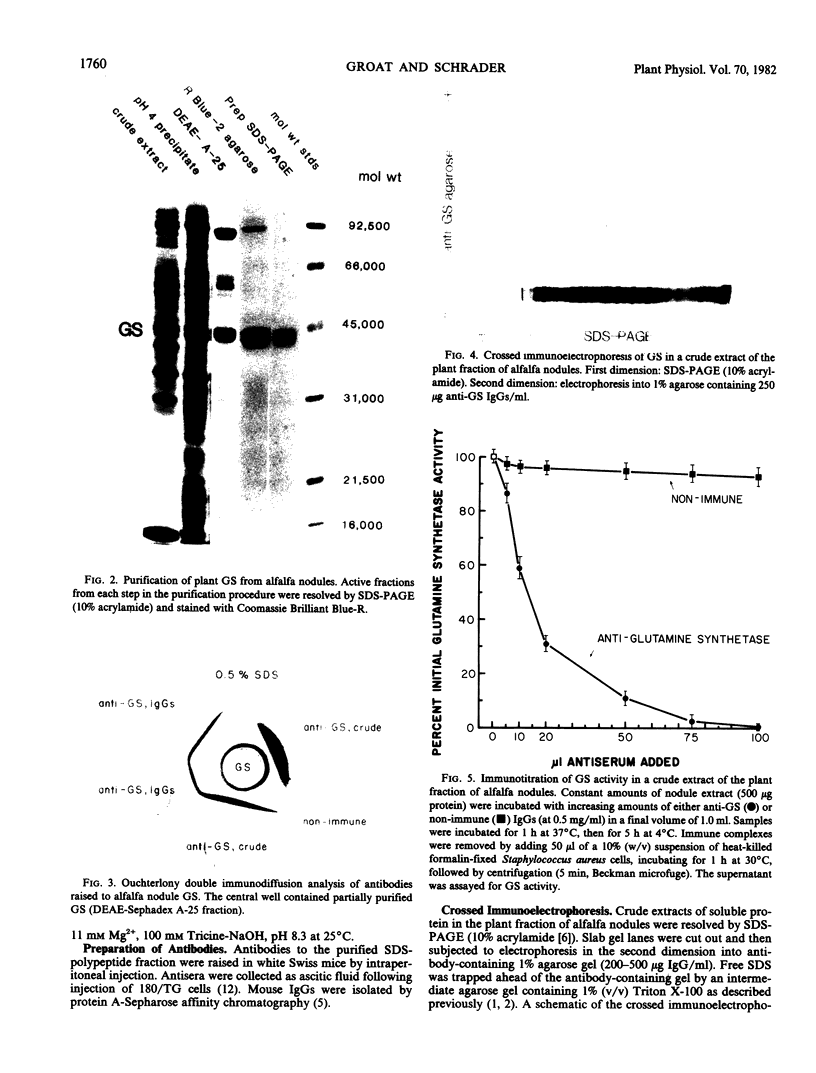
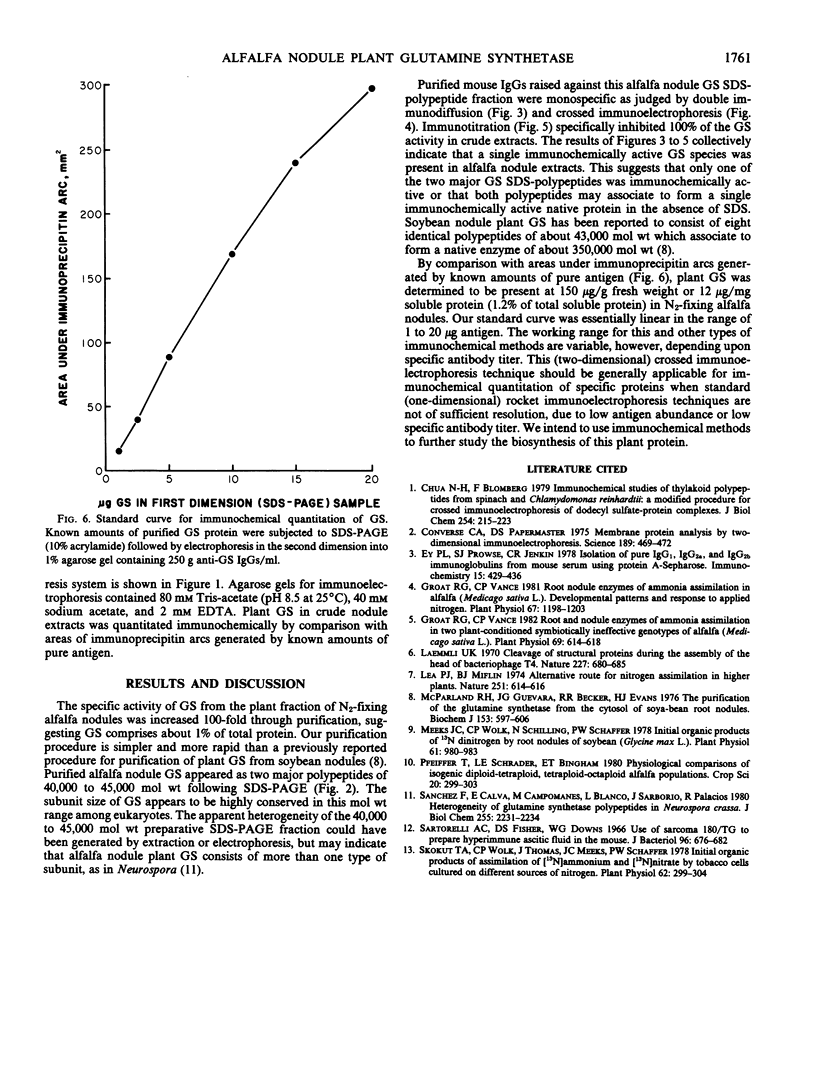
Host plant glutamine synthetase (GS) has been purified 100-fold from N2-fixing alfalfa (Medicago sativa L.) nodules by a new procedure involving preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as a final step. An SDS-polypeptide fraction corresponding to plant GS was identified and consisted of two major polypeptides of 40,000 to 45,000 molecular weight. Antibodies to the SDS-polypeptide fraction were raised in mice by intraperitoneal injection, and antisera were collected as ascitic fluid. Crude extracts of soluble protein from the plant fraction of nodules were resolved by SDS-PAGE and then subjected to electrophoresis in the second dimension into antibody-containing agarose gel. A single immunochemically active protein species was observed using this crossed immunoelectrophoresis method, even though both major GS SDS-polypeptides were apparently resolved in the first (SDS-PAGE) dimension. Plant GS protein in crude nodule extracts was quantitated immunochemically by comparison with immunoprecipitin arcs of similarly treated amounts of pure antigen. Using this technique, it was determined that plant GS was present at 150 micrograms per gram fresh weight or 1.2% of total plant soluble protein in N2-fixing alfalfa nodules.
Results suggest that alfalfa nodule plant GS consists of two major subunit polypeptides, but only a single immunochemically active native protein was observed. The crossed immunoelectrophoresis procedure described here should be generally applicable for immunochemical detection of lower abundance components of crude plant extracts.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunetti N., Hageman R. H. Comparison of in Vivo and in Vitro Assays of Nitrate Reductase in Wheat (Triticum aestivum L.) Seedlings. Plant Physiol. 1976 Oct;58(4):583–587. doi: 10.1104/pp.58.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Converse C. A., Papermaster D. S. Membrane protein analysis by two-dimensional immunoelectrophoresis. Science. 1975 Aug 8;189(4201):469–472. doi: 10.1126/science.1154021. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.) : DEVELOPMENTAL PATTERNS AND RESPONSE TO APPLIED NITROGEN. Plant Physiol. 1981 Jun;67(6):1198–1203. doi: 10.1104/pp.67.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root and Nodule Enzymes of Ammonia Assimilation in Two Plant-Conditioned Symbiotically Ineffective Genotypes of Alfalfa (Medicago sativa L.). Plant Physiol. 1982 Mar;69(3):614–618. doi: 10.1104/pp.69.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. S., Gandhi A. P., Sawhney S. K., Naik M. S. Inhibitor of nitrate reductase in the roots of rice seedlings and its effect on the enzyme activity in the presence of NADH. Biochim Biophys Acta. 1974 May 20;350(1):162–170. doi: 10.1016/0005-2744(74)90214-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Leong C. C., Shen T. C. Nitrate reductase inhibitor of rice plants. Biochim Biophys Acta. 1980 Mar 14;612(1):245–252. doi: 10.1016/0005-2744(80)90298-3. [DOI] [PubMed] [Google Scholar]
- McParland R. H., Guevara J. G., Becker R. R., Evans H. J. The purification and properties of the glutamine synthetase from the cytosol of Soya-bean root nodules. Biochem J. 1976 Mar 1;153(3):597–606. doi: 10.1042/bj1530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Schilling N., Shaffer P. W. Initial Organic Products of Fixation of [N]Dinitrogen by Root Nodules of Soybean (Glycine max). Plant Physiol. 1978 Jun;61(6):980–983. doi: 10.1104/pp.61.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Shen T. C. The induction of nitrate reductase and the preferential assimilation of ammonium in germinating rice seedlings. Plant Physiol. 1969 Nov;44(11):1650–1655. doi: 10.1104/pp.44.11.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J. H., Kennedy J. A., Dalling M. J. In Vitro Stability of Nitrate Reductase from Wheat Leaves: III. Isolation and Partial Characterization of a Nitrate Reductase-inactivating Factor. Plant Physiol. 1979 Oct;64(4):640–645. doi: 10.1104/pp.64.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokut T. A., Wolk C. P., Thomas J., Meeks J. C., Shaffer P. W. Initial organic products of assimilation of [N]ammonium and [N]nitrate by tobacco cells cultured on different sources of nitrogen. Plant Physiol. 1978 Aug;62(2):299–304. doi: 10.1104/pp.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez F., Calva E., Campomanes M., Blanco L., Guzmán J., Saborío J. L., Palacios R. Heterogeneity of glutamine synthetase polypeptides in Neurospora crassa. J Biol Chem. 1980 Mar 25;255(6):2231–2234. [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]