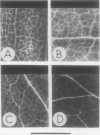
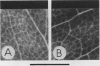
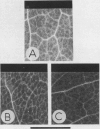
Abstract
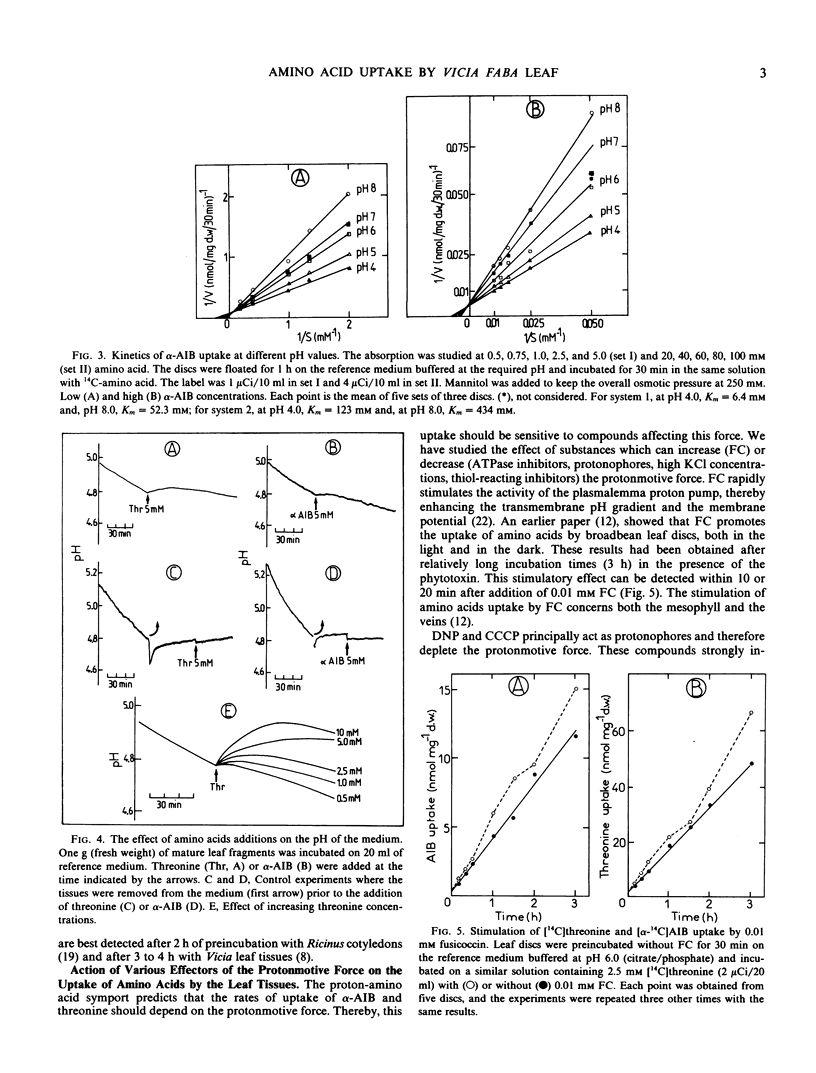
The uptake of [U-14C]threonine and of (α-14C]aminoisobutyrate (α-AIB) by Vicia faba leaf discs is strongly pH dependent (optimum: pH 4.0) and exhibits biphasic saturation kinetics. Kinetics of α-AIB uptake at different pH values indicate that acidic pH values decrease the Km of the carriers while the maximal velocity remains nearly unaffected. Similar results were obtained for both system 1 (from 0.5 to 5 millimolar) and system 2 (from 20 to 100 millimolar).
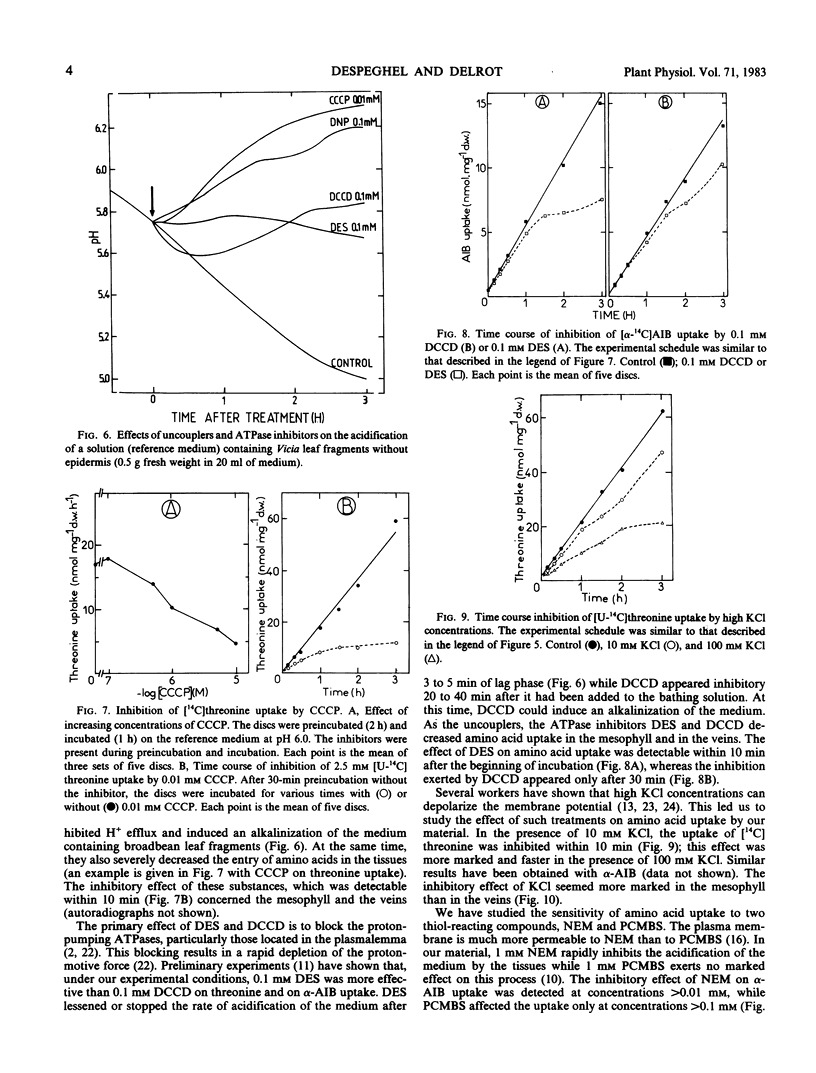
After addition of amino acids to a medium containing leaf fragments, alkalinizations depending both on the amino acid added and on its concentration have been recorded.
The effects of compounds which increase (fusicoccin) or decrease (uncouplers, ATPase inhibitors, high KCl concentrations) the protonmotive force were studied both on the acidification of the medium and on amino acid uptake by the tissues. There is a close relationship between the time required for the effect of these compounds on the acidification and that needed for inhibition of uptake.
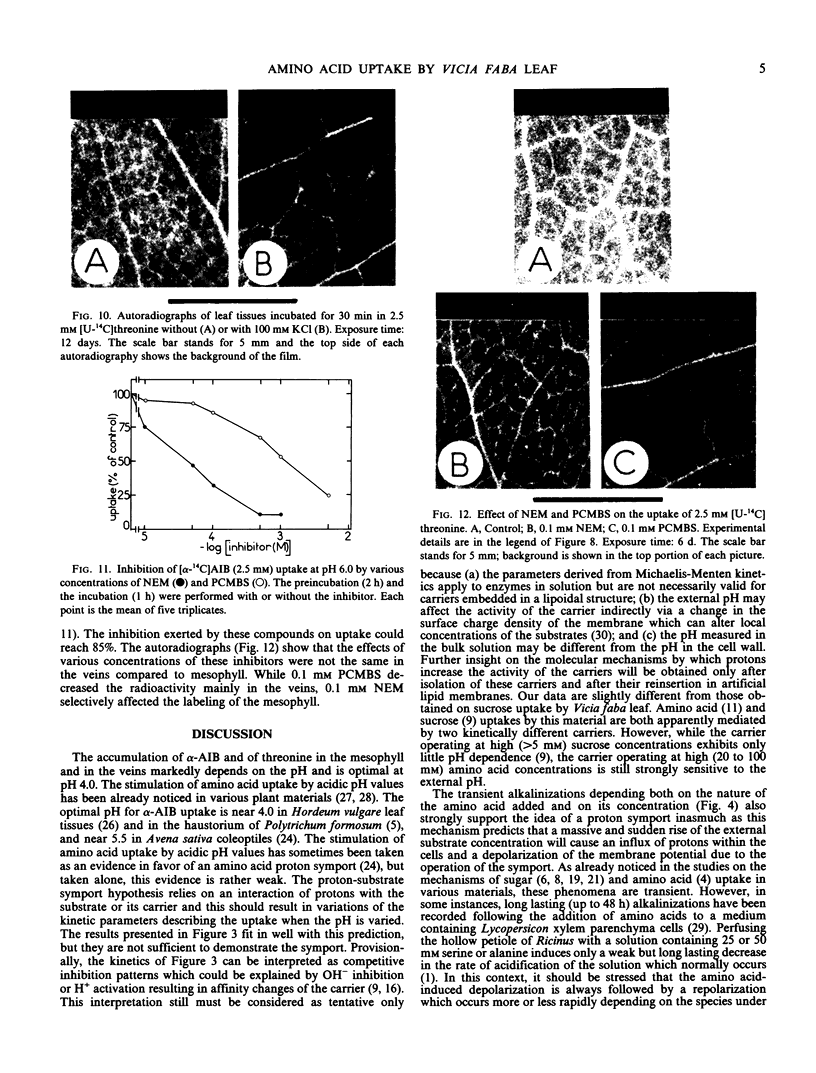
Studies with thiol inhibitors show that 0.1 millimolar N-ethylmaleimide preferentially inhibits uptake by the mesophyll whereas 0.1 millimolar parachloromercuribenzenesulfonate affects rather uptake by the veins.
New evidence was found which added to the electrophysiological data already supporting the occurrence of proton amino acid symport in leaf tissues, particularly in the veins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S., Bonnemain J. L. Involvement of Protons as a Substrate for the Sucrose Carrier during Phloem Loading in Vicia faba Leaves. Plant Physiol. 1981 Mar;67(3):560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S. Proton Fluxes Associated with Sugar Uptake in Vicia faba Leaf Tissues. Plant Physiol. 1981 Sep;68(3):706–711. doi: 10.1104/pp.68.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Lüttge U. Membrane Potential Changes Related to Active Transport of Glycine in Lemna gibba G1. Plant Physiol. 1980 May;65(5):1004–1008. doi: 10.1104/pp.65.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine transport into cultured tobacco cells. Plant Physiol. 1977 Dec;60(6):807–811. doi: 10.1104/pp.60.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Schrader L. E., Jung D. M. Energy-dependent Loading of Amino Acids and Sucrose into the Phloem of Soybean. Plant Physiol. 1979 Oct;64(4):546–550. doi: 10.1104/pp.64.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Role of calcium in serine transport into tobacco cells. Plant Physiol. 1978 Dec;62(6):941–948. doi: 10.1104/pp.62.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Nałecz M. J. Surface change of biological membranes as a possible regulator of membrane-bound enzymes. Eur J Biochem. 1979 Feb 15;94(1):99–107. doi: 10.1111/j.1432-1033.1979.tb12876.x. [DOI] [PubMed] [Google Scholar]