Abstract
A mechanical isolation procedure was developed to study the respiratory properties of mitochondria from the mesophyll and bundle sheath tissue of Panicum miliaceum, a NAD-malic enzyme C4 plant. A mesophyll fraction and a bundle sheath fraction were obtained from young leaves by differential mechanical treatment. The purity of both fractions was about 80%, based on analysis of the cross-contamination of ribulose bisphosphate carboxylase activity and phosphoenolpyruvate carboxylase activity.
Mitochondria were isolated from the two fractions by differential centrifugation and Percoll density gradient centrifugation. The enrichment of mitochondria relative to chloroplast material was about 75-fold in both preparations.
Both types of mitochondria oxidized NADH and succinate with respiratory control. Malate oxidation in mesophyll mitochondria was sensitive to KCN and showed good respiratory control. In bundle sheath mitochondria, malate oxidation was largely insensitive to KCN and showed no respiratory control. The oxidation was strongly inhibited by salicylhydroxamic acid, showing that the alternative oxidase was involved. The bundle sheath mitochondria of this type of C4 species contribute to C4 photosynthesis through decarboxylation of malate. Malate oxidation linked to an uncoupled, alternative pathway may allow decarboxylation to proceed without the restraints which might occur via coupled electron flow through the cytochrome chain.
Full text
PDF

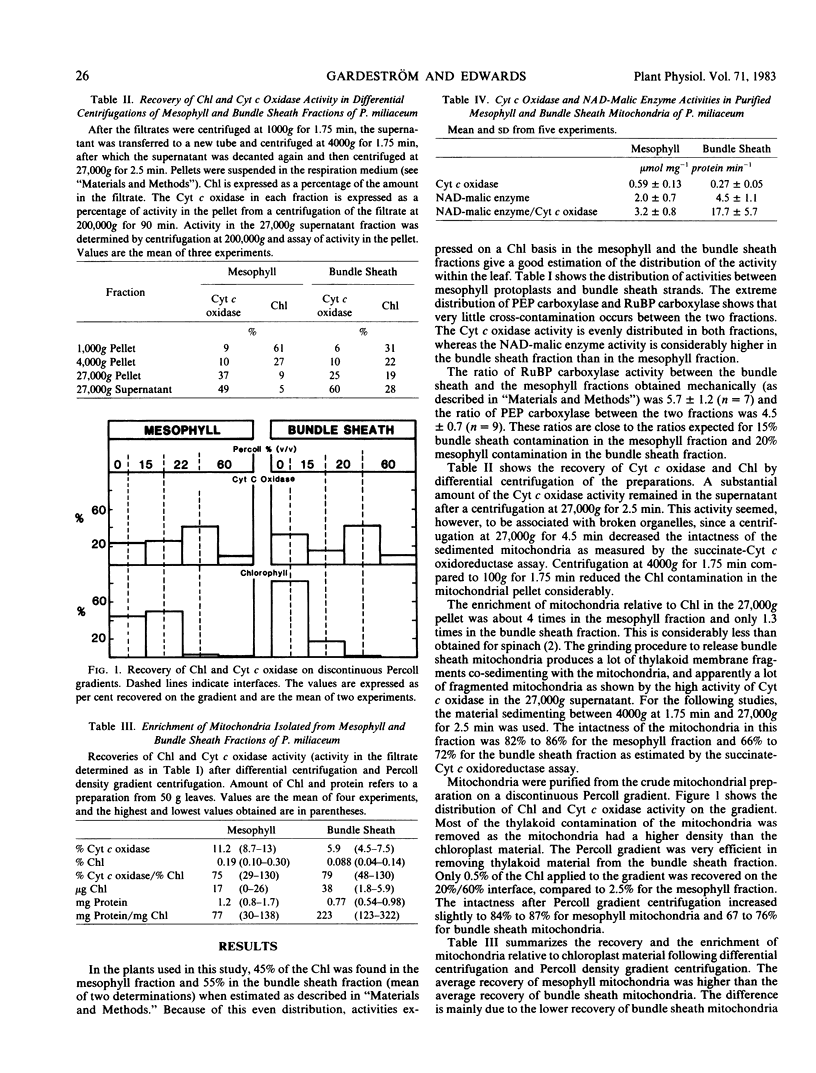
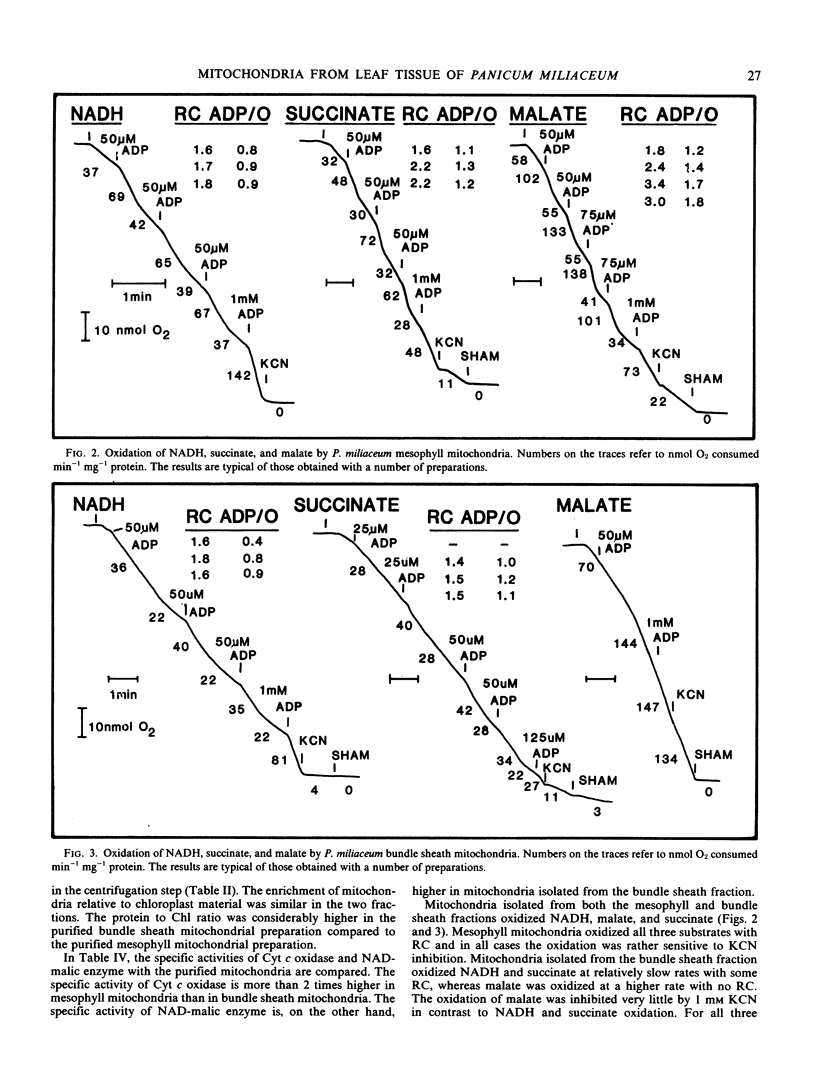


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Bergman A., Gardeström P., Ericson I. Method to Obtain a Chlorophyll-free Preparation of Intact Mitochondria from Spinach Leaves. Plant Physiol. 1980 Sep;66(3):442–445. doi: 10.1104/pp.66.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E. Isolation of Intact and Functional Chloroplasts from Mesophyll and Bundle Sheath Protoplasts of the C(4) Plant Panicum miliaceum. Plant Physiol. 1979 May;63(5):821–827. doi: 10.1104/pp.63.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Haas R., Wrage K., Heinz E. Phospholipid composition of chlorophyll-free mitochondria isolated via protoplasts from oat mesophyll cells. Hoppe Seylers Z Physiol Chem. 1981 Aug;362(8):1069–1078. doi: 10.1515/bchm2.1981.362.2.1069. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Tsuzuki M., Edwards G. E. Determination of NAD Malic Enzyme in Leaves of C(4) Plants : EFFECTS OF MALATE DEHYDROGENASE AND OTHER FACTORS. Plant Physiol. 1982 Feb;69(2):483–491. doi: 10.1104/pp.69.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C., Dench J. E., Hall D. O., Moore A. L. Separation of mitochondria from contaminating subcellular structures utilizing silica sol gradient centrifugation. Plant Physiol. 1979 Jul;64(1):150–153. doi: 10.1104/pp.64.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Mitochondria as a site of C4 acid decarboxylation in C4-pathway photosynthesis. Arch Biochem Biophys. 1975 Apr;167(2):687–696. doi: 10.1016/0003-9861(75)90513-5. [DOI] [PubMed] [Google Scholar]
- Keister D. L., Minton N. J. Energy-linked reactions in photosynthetic bacteria. VI. Inorganic pyrophosphate-driven ATP synthesis in Rhodospirillum rubrum. Arch Biochem Biophys. 1971 Nov;147(1):330–338. doi: 10.1016/0003-9861(71)90341-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Douce R. Effect of bicarbonate and oxaloacetate on malate oxidation by spinach leaf mitochondria. Biochim Biophys Acta. 1980 Feb 8;589(2):176–189. doi: 10.1016/0005-2728(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Douce R. Oxydation du malate, du NADH et de la glycine par les mitochondries de plantes en C3 et C4. C R Acad Sci Hebd Seances Acad Sci D. 1977 Oct 10;285(8):881–884. [PubMed] [Google Scholar]
- Nishimura M., Douce R., Akazawa T. Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol. 1982 Apr;69(4):916–920. doi: 10.1104/pp.69.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P., Moreau F., Lance C. Malate Oxidation in Plant Mitochondria via Malic Enzyme and the Cyanide-insensitive Electron Transport Pathway. Plant Physiol. 1980 Sep;66(3):457–462. doi: 10.1104/pp.66.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


