Abstract
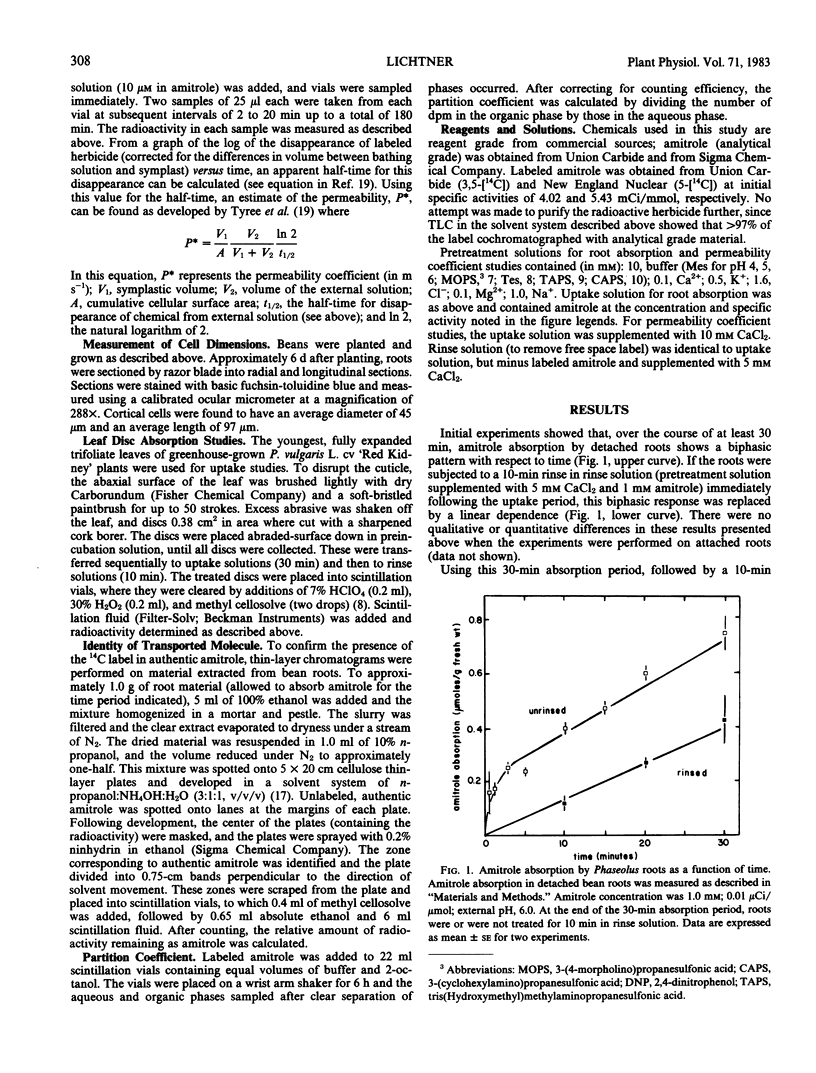
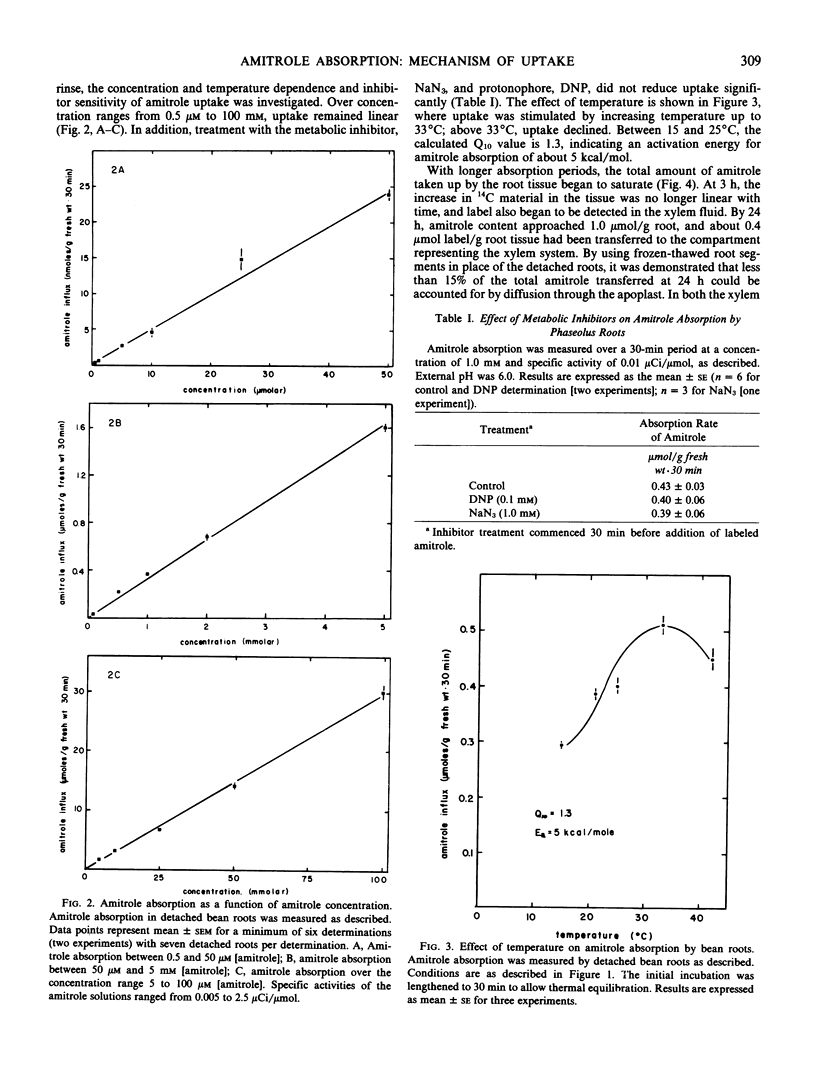
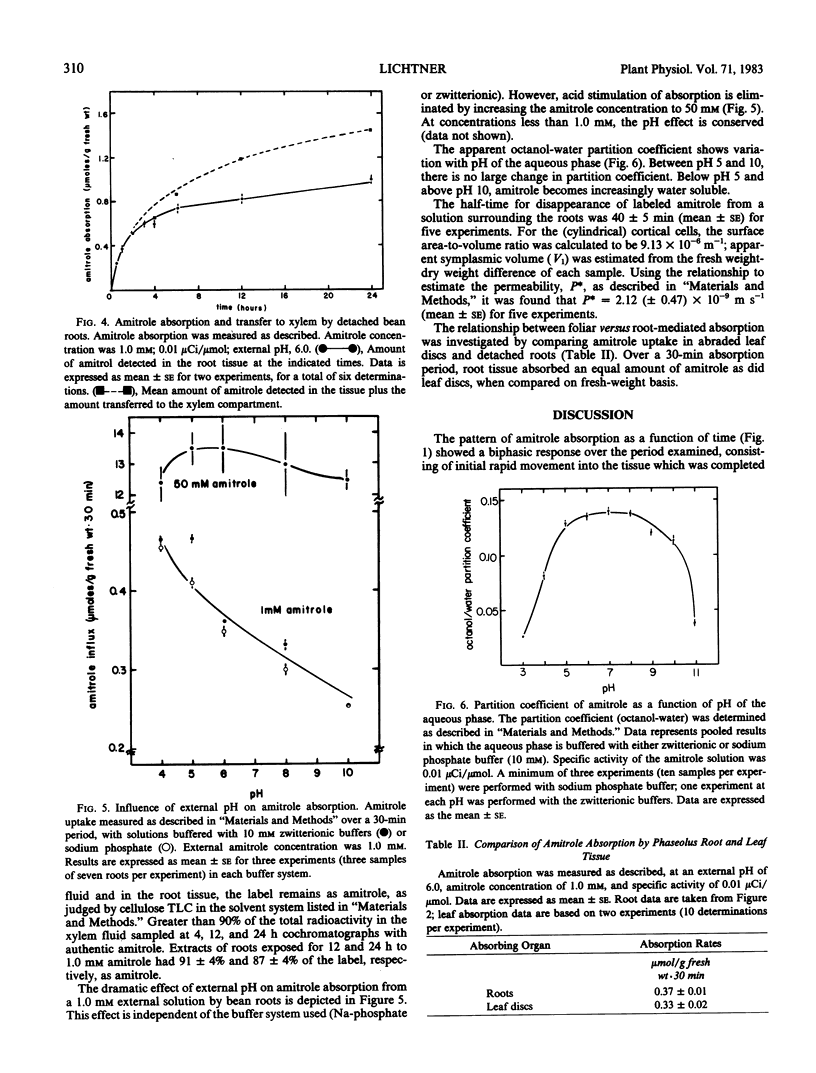
The mechanism of transport of the herbicide 3-amino-1,2,4-triazole (amitrole) into Phaseolus vulgaris roots appears to be passive, as judged by the effect of temperature (Q10 = 1.3 between 15 and 25°C) and the lack of sensitivity to metabolic inhibition afforded by 2,4-dinitrophenol and NaN3. Amitrole absorption is a linear function of external concentration over several orders of magnitude and, thus, is not facilitated by a carrier mechanism. The absorption of amitrole is sensitive to external pH, being stimulated under acid conditions. This stimulation of amitrole absorption is seen at low (≤1 millimolar) amitrole concentrations, but not at high (50 millimolar) amitrole levels. While the apparent octanol-water partition coefficient varies with the pH of the aqueous phase, there is no clear correspondence between absorption and the apparent partition coefficient. Roots do not accumulate amitrole above concentration equilibrium; however, at a time when the net amitrole content of the root tissue begins to saturate, amitrole can be detected in the xylem stream. On a fresh-weight basis, amitrole absorption by roots is equal to that accomplished by trifoliate-leaf tissue. An estimate of the permeability coefficient (according to the analysis of Tyree et al. 1979 Plant Physiol 63: 367-374) suggests that amitrole possesses near-optimal permeability for an ambimobile solute, on the order of 2.12 (± 0.47) × 10−9 meters per second.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donnalley W. F., Ries S. K. Amitrole Translocation in Agropyron repens Increased by the Addition of Ammonium Thiocyanate. Science. 1964 Jul 31;145(3631):497–498. doi: 10.1126/science.145.3631.497. [DOI] [PubMed] [Google Scholar]
- Dreyer S. A., Seymour V., Cleland R. E. Low proton conductance of plant cuticles and its relevance to the Acid-growth theory. Plant Physiol. 1981 Sep;68(3):664–667. doi: 10.1104/pp.68.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougler J. A., Geiger D. R. Uptake and distribution of N-phosphonomethylglycine in sugar beet plants. Plant Physiol. 1981 Sep;68(3):668–672. doi: 10.1104/pp.68.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. R., McDaniel C. N. Transport of the herbicide 3-amino-1,2,4-triazole by cultured tobacco cells and leaf protoplasts. Plant Physiol. 1982 Jun;69(6):1382–1386. doi: 10.1104/pp.69.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T. A simple theory regarding ambimobility of xenobiotics with special reference to the nematicide, oxamyl. Plant Physiol. 1979 Feb;63(2):367–374. doi: 10.1104/pp.63.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]


