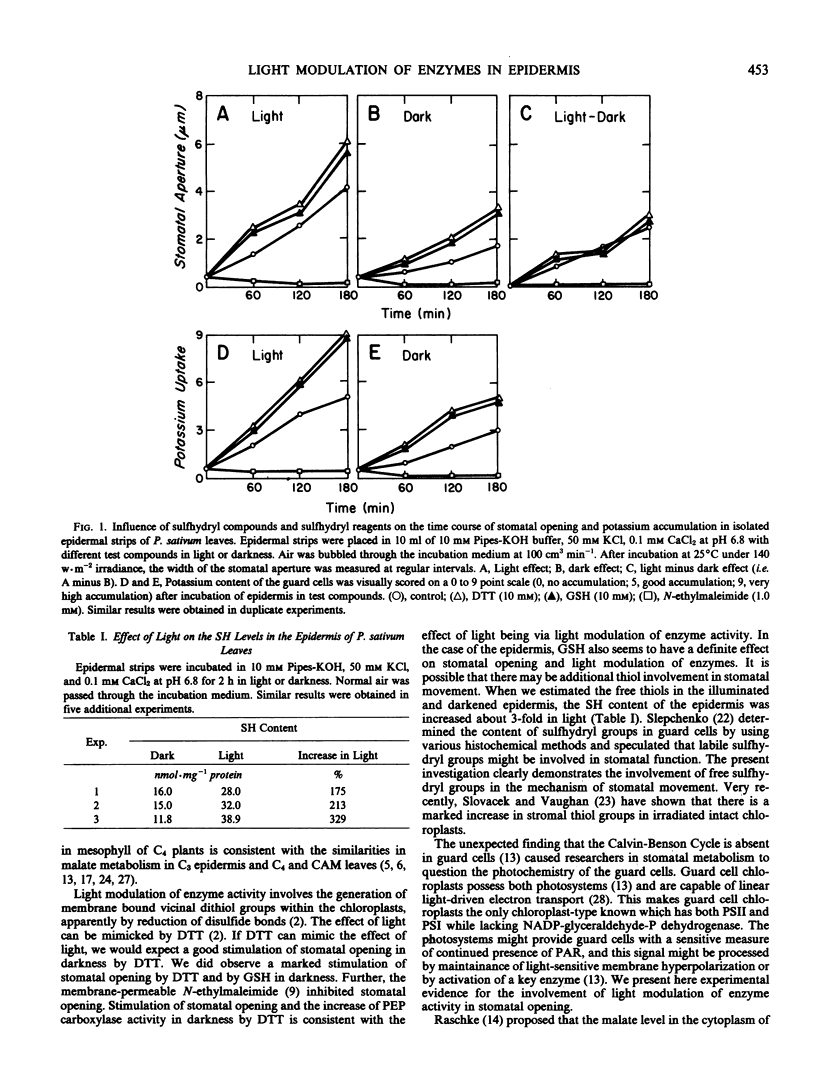
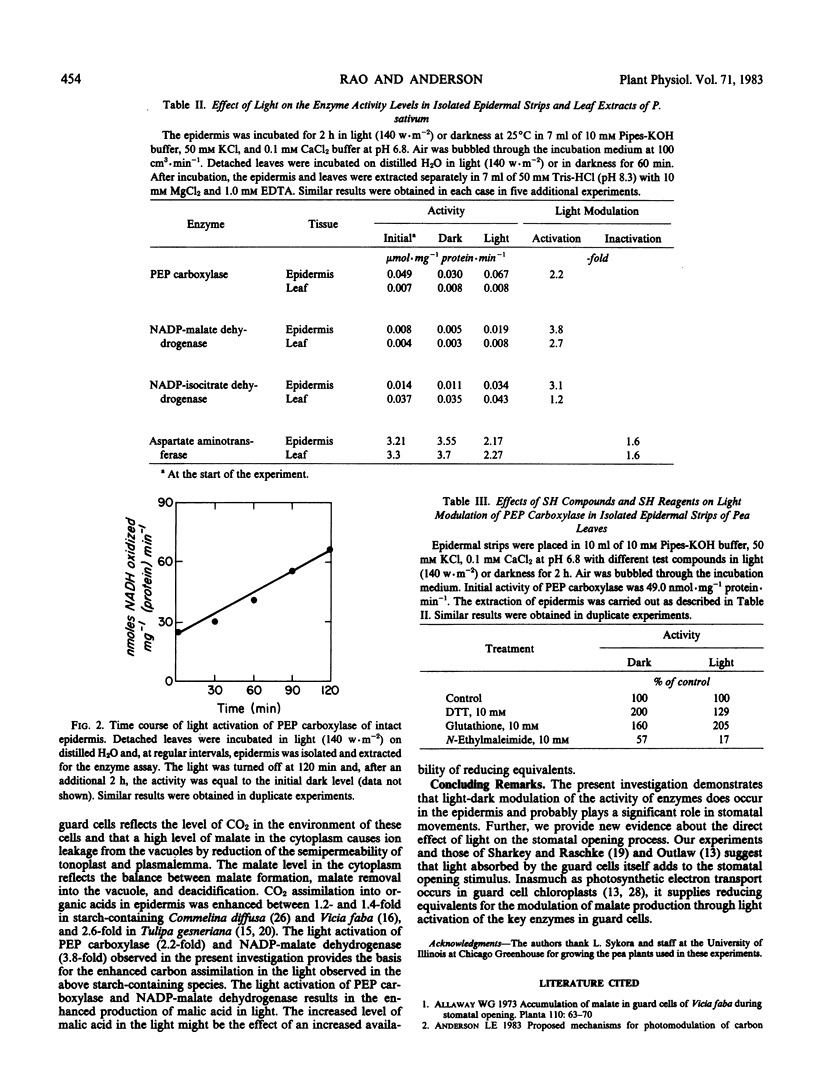
Abstract
New evidence is provided regarding the direct effect of light on stomatal opening in the epidermis of the pea (Pisum sativum L. var Little Marvel) leaf. Light modulates the activity of a number of key enzymes involved in stomatal metabolism. When isolated epidermal strips are illuminated, phosphoenolpyruvate carboxylase, NADP-malate dehydrogenase, and NADP-isocitrate dehydrogenase are activated; and aspartate aminotransferase is inactivated. Sulfhydryl compounds, dithiothreitol and glutathione, enhance stomatal opening in epidermal strips both in light or darkness while the sulfhydryl reagent N-ethylmaleimide inhibits, indicating the possible involvement of sulfhydryl groups in stomatal movements. Further, light treatment increases measureable thiol levels in the epidermis about 3-fold. These results suggest that light modulation of enzymes in the epidermis may play a significant role in the mechanism of stomatal movement.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sharkey T. D., Raschke K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiol. 1981 Jul;68(1):33–40. doi: 10.1104/pp.68.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R. The photoactivation of a phosphopyruvate synthase in leaves of Amaranthus palmeri. Biochem Biophys Res Commun. 1968 Mar 12;30(5):483–488. doi: 10.1016/0006-291x(68)90077-6. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Vaughn S. Chloroplast sulfhydryl groups and the light activation of fructose-1,6-bisphosphatase. Plant Physiol. 1982 Oct;70(4):978–981. doi: 10.1104/pp.70.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


