Abstract
Young bean plants (Phaseolus vulgaris L. var Saxa) were fed with three different types of inorganic nitrogen, after being grown on nitrogen-free nutrient solution for 8 days. The pattern of 14CO2 fixation was investigated in photosynthesizing primary leaf discs of 11-day-old plants (3 days with nitrogen source) and in a pulse-chase experiment in 13-day-old plants (5 days with nitrogen source).
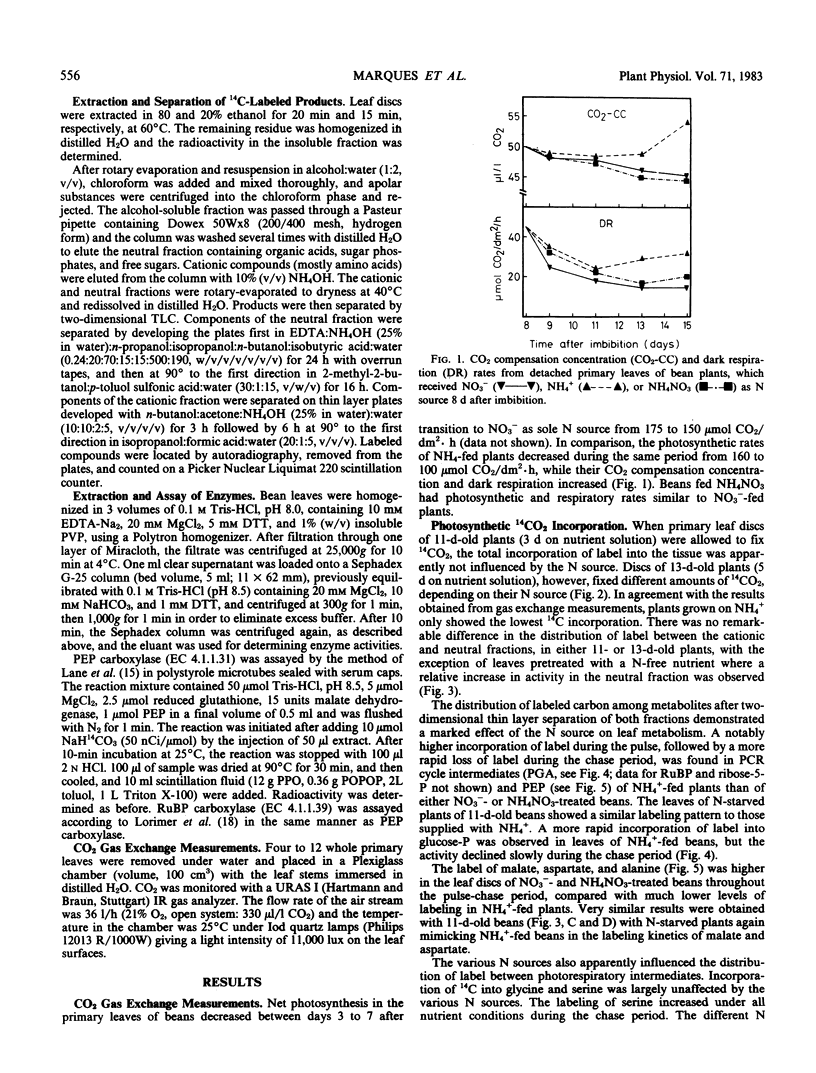
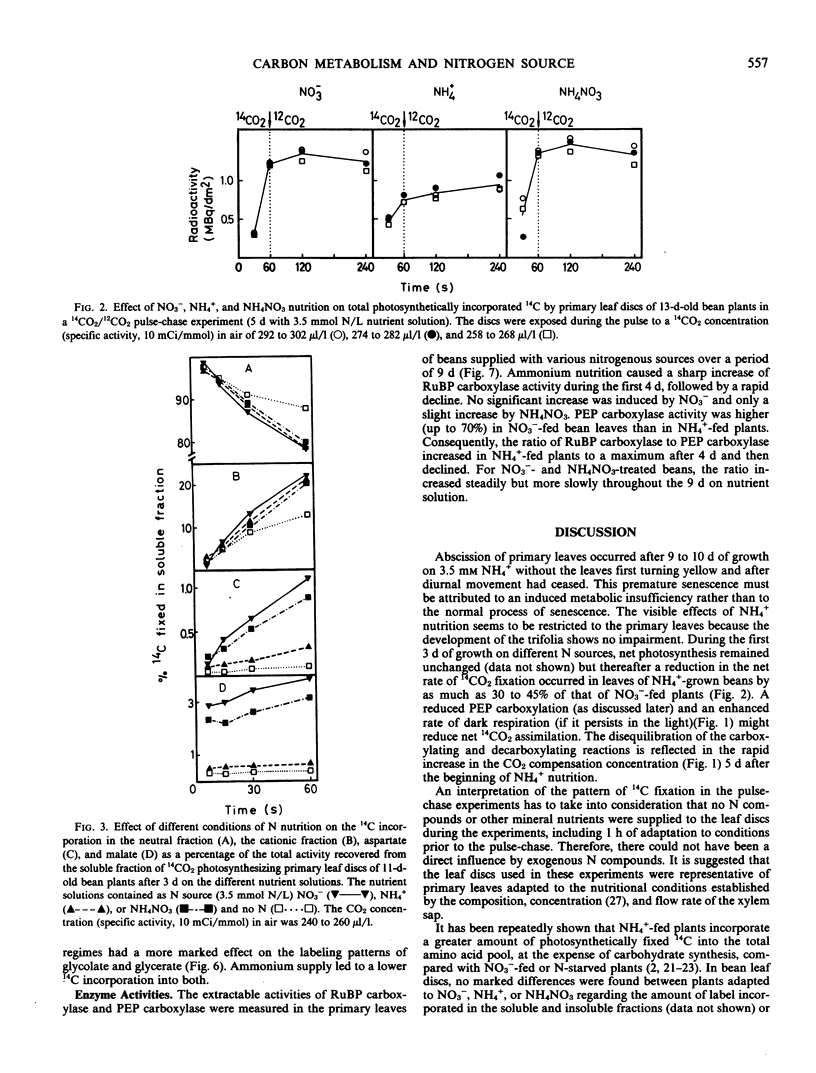
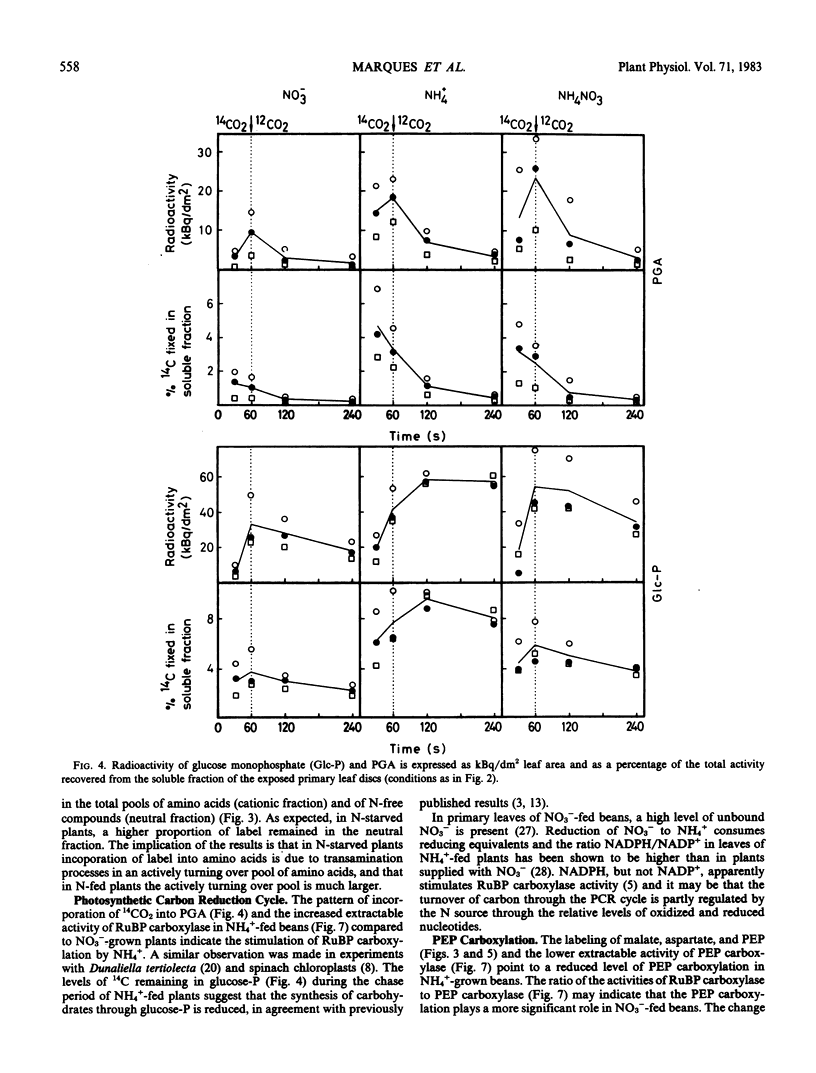
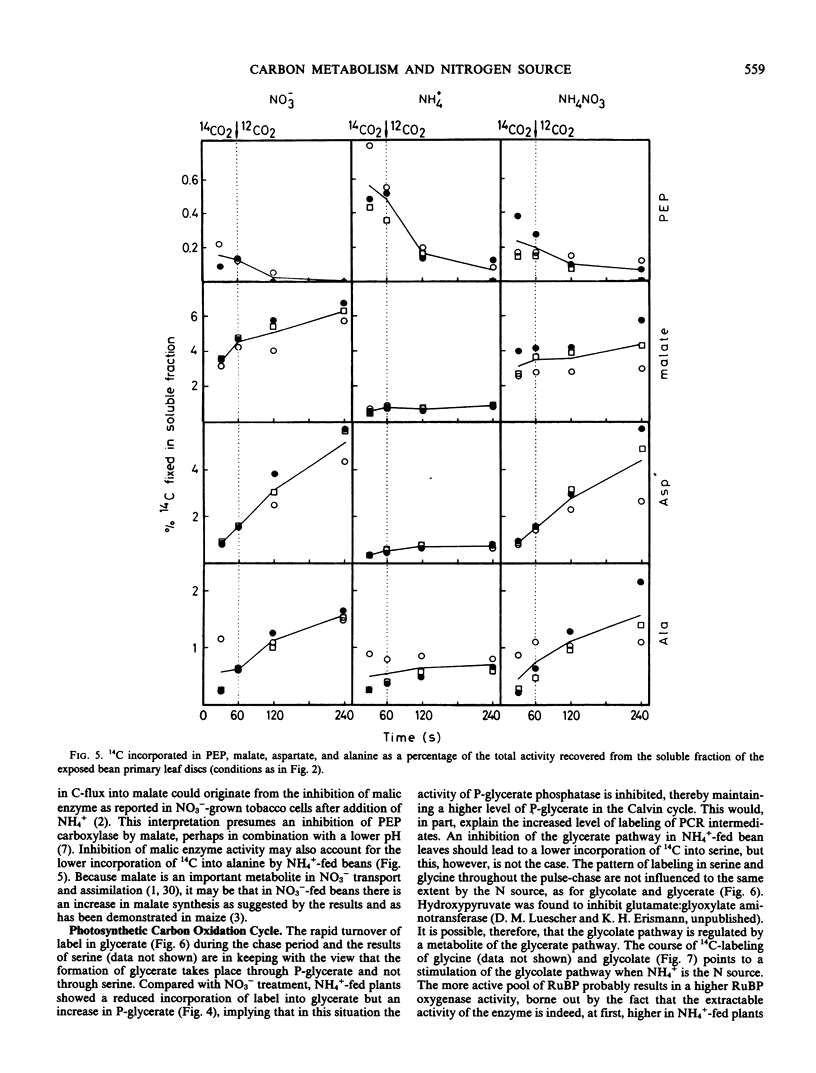
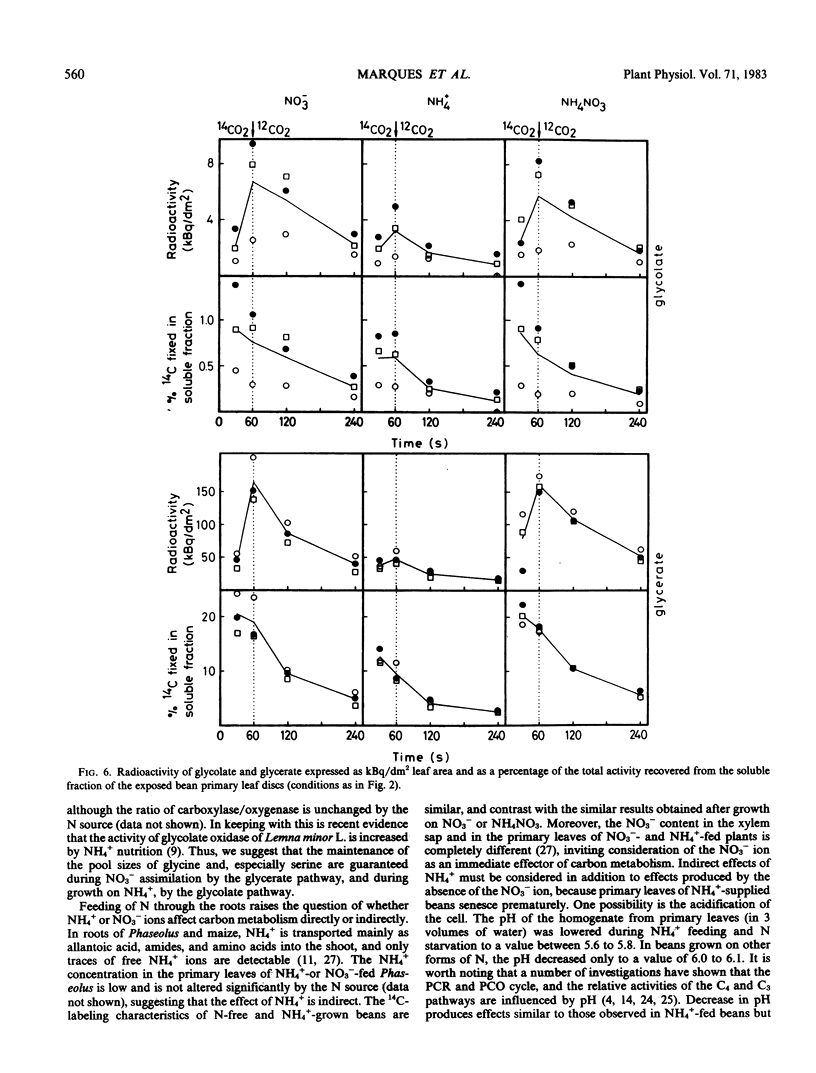
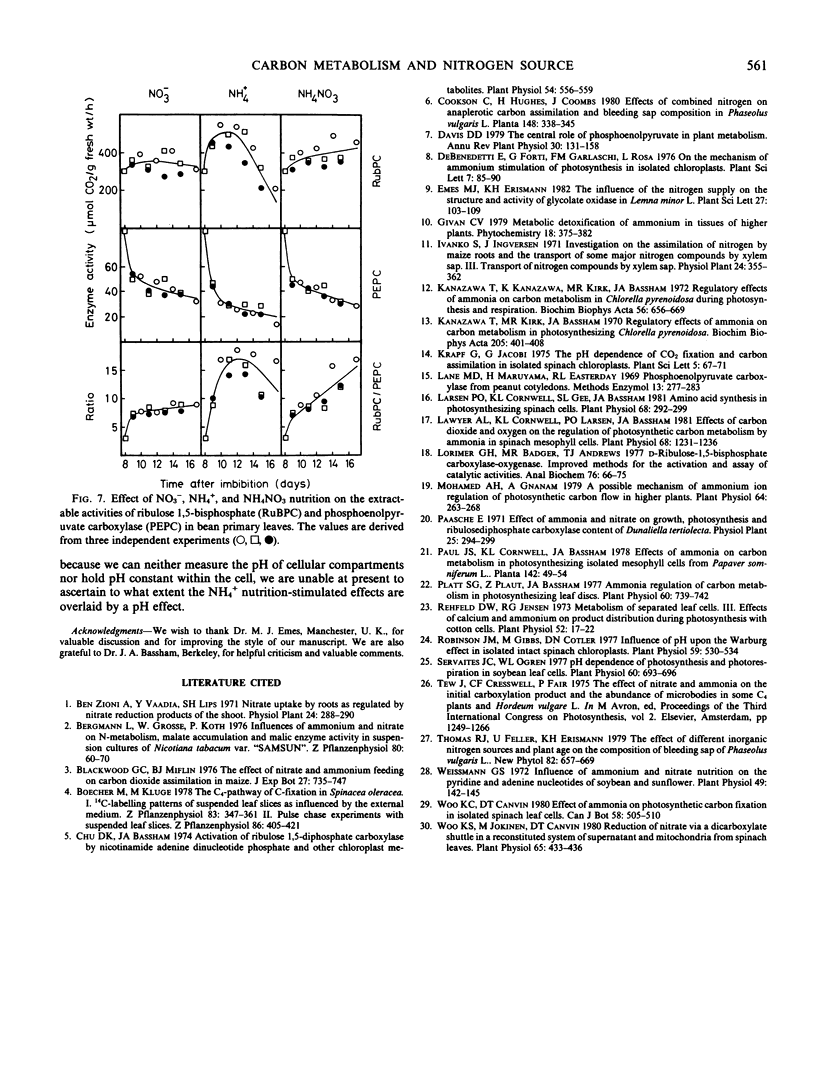
Ammonium caused, in contrast to nitrate nutrition, a higher level of 14C incorporation into sugar phosphates but a lower incorporation of label into malate, glycolate, glycerate, aspartate, and alanine. The labeling kinetics of glycine and serine were little changed by the nitrogen source. Ammonium feeding also produced an increase in the ratio of extractable activities of ribulose-1,5-bisphosphate carboxylase to phosphoenolpyruvate carboxylase and an increase in dark respiration and the CO2 compensation concentration. Net photosynthesis was higher in plants assimilating nitrate.
The results point to stimulated turnover of the photosynthetic carbon reduction cycle metabolites, reduced phosphoenolpyruvate carboxylation, and altered turnover rates within the photosynthetic carbon oxidation cycle in ammonium-fed plants. Mechanisms of the regulation of primary carbon metabolism are proposed and discussed.
Full text
PDF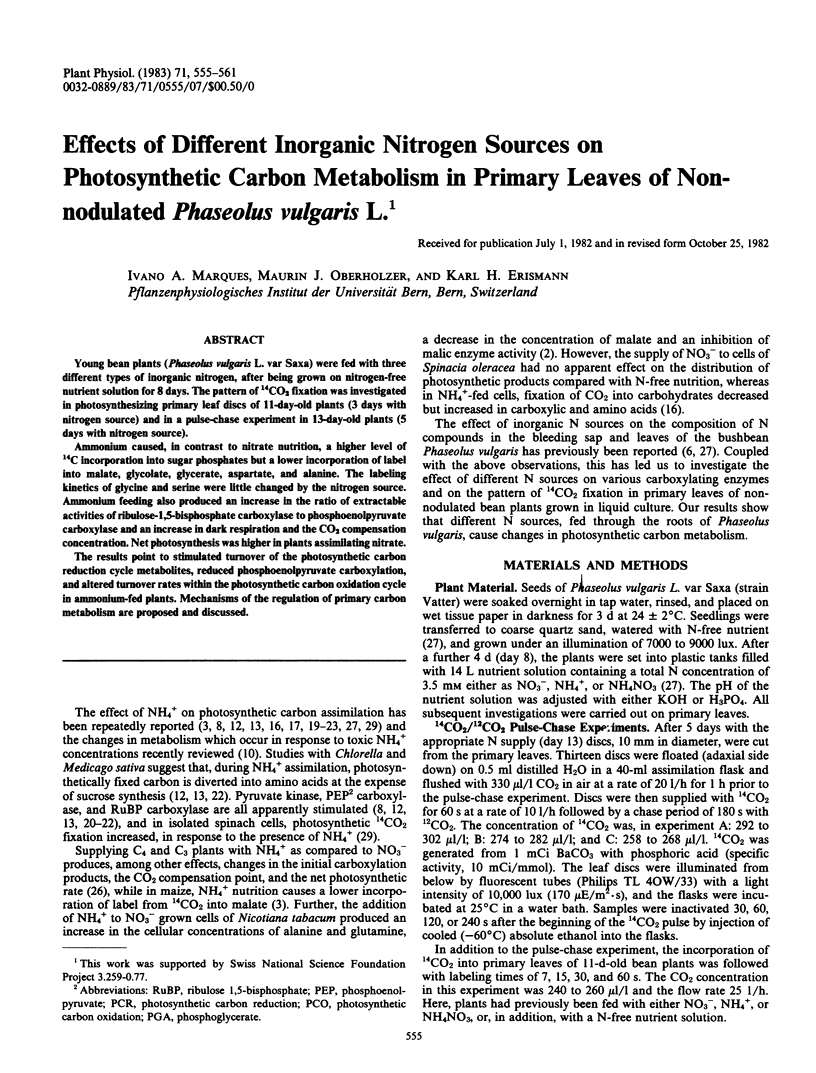
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu D. K., Bassham J. A. Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine dinucleotide phosphate and other chloroplast metabolites. Plant Physiol. 1974 Oct;54(4):556–559. doi: 10.1104/pp.54.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kanazawa K., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in Chlorella pyrenoidosa during photosynthesis and respiration. Biochim Biophys Acta. 1972 Mar 16;256(3):656–669. doi: 10.1016/0005-2728(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Larsen P. O., Cornwell K. L., Gee S. L., Bassham J. A. Amino Acid Synthesis in Photosynthesizing Spinach Cells : EFFECTS OF AMMONIA ON POOL SIZES AND RATES OF LABELING FROM CO(2). Plant Physiol. 1981 Aug;68(2):292–299. doi: 10.1104/pp.68.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer A. L., Cornwell K. L., Larsen P. O., Bassham J. A. Effects of carbon dioxide and oxygen on the regulation of photosynthetic carbon metabolism by ammonia in spinach mesophyll cells. Plant Physiol. 1981 Dec;68(6):1231–1236. doi: 10.1104/pp.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Gnanam A. A possible mechanism of ammonium ion regulation of photosynthetic carbon flow in higher plants. Plant Physiol. 1979 Aug;64(2):263–268. doi: 10.1104/pp.64.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Jensen R. G. Metabolism of Separated Leaf Cells: III. Effects of Calcium and Ammonium on Product Distribution During Photosynthesis with Cotton Cells. Plant Physiol. 1973 Jul;52(1):17–22. doi: 10.1104/pp.52.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M., Cotler D. N. Influence of pH upon the Warburg Effect in Isolated Intact Spinach Chloroplasts: I. Carbon Dioxide Photoassimilation and Glycolate Synthesis. Plant Physiol. 1977 Apr;59(4):530–534. doi: 10.1104/pp.59.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. pH Dependence of Photosynthesis and Photorespiration in Soybean Leaf Cells. Plant Physiol. 1977 Nov;60(5):693–696. doi: 10.1104/pp.60.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman G. S. Influence of ammonium and nitrate nutrition on the pyridine and adenine nucleotides of soybean and sunflower. Plant Physiol. 1972 Feb;49(2):142–145. doi: 10.1104/pp.49.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Jokinen M., Canvin D. T. Reduction of Nitrate via a Dicarboxylate Shuttle in a Reconstituted System of Supernatant and Mitochondria from Spinach Leaves. Plant Physiol. 1980 Mar;65(3):433–436. doi: 10.1104/pp.65.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


