Abstract
The effect of light and CO2 on both the endogenous and 1-aminocyclopropane-1-carboxylic acid (ACC)-dependent ethylene evolution from metabolically active detached leaves and leaf discs of Gomphrena globosa L. is reported. Treatment with varying concentrations of ACC did not appear to inhibit photosynthesis, respiration, or stomatal behavior. In all treatments, more ethylene was released into a closed flask from ACC-treated tissue, but the pattern of ethylene release with respect to light/dark/CO2 treatments was the same.
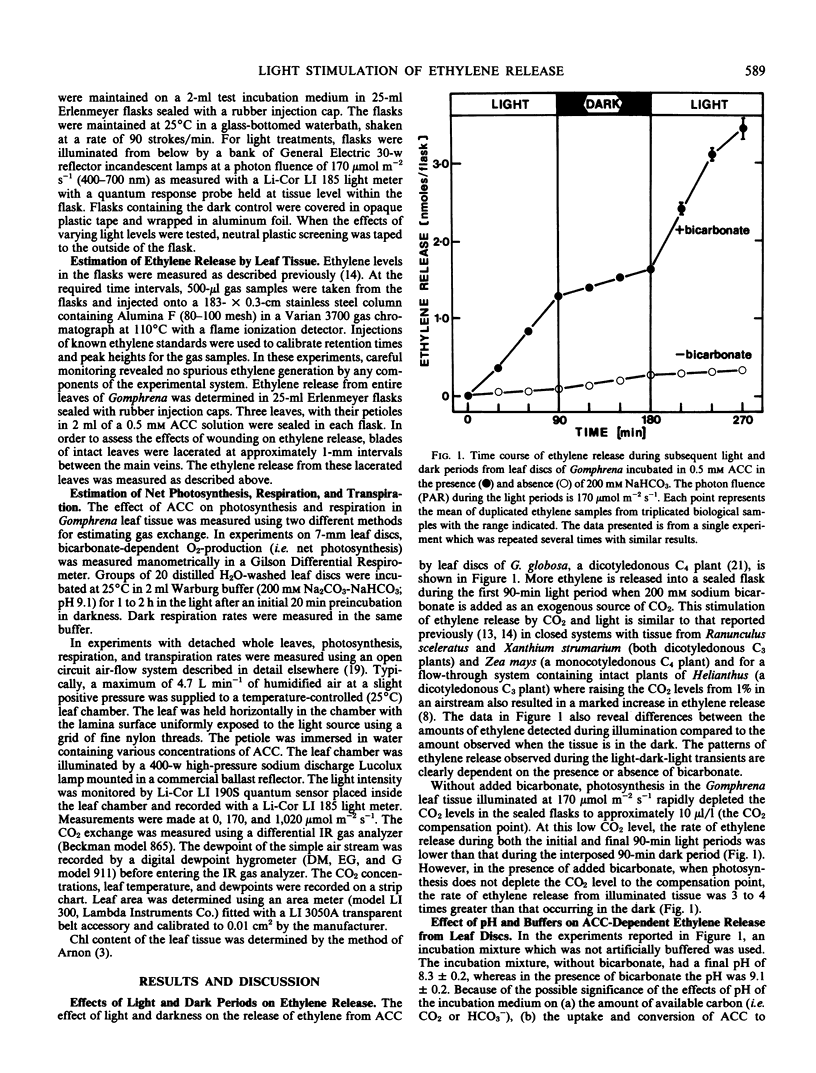
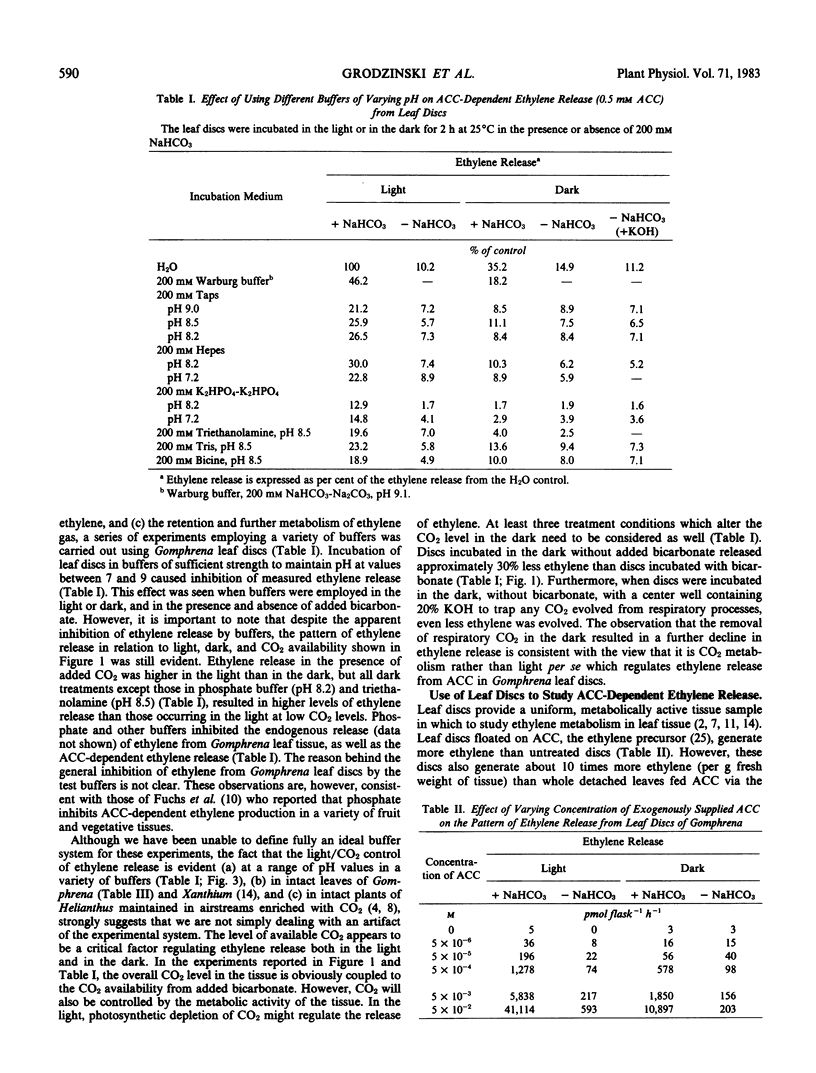
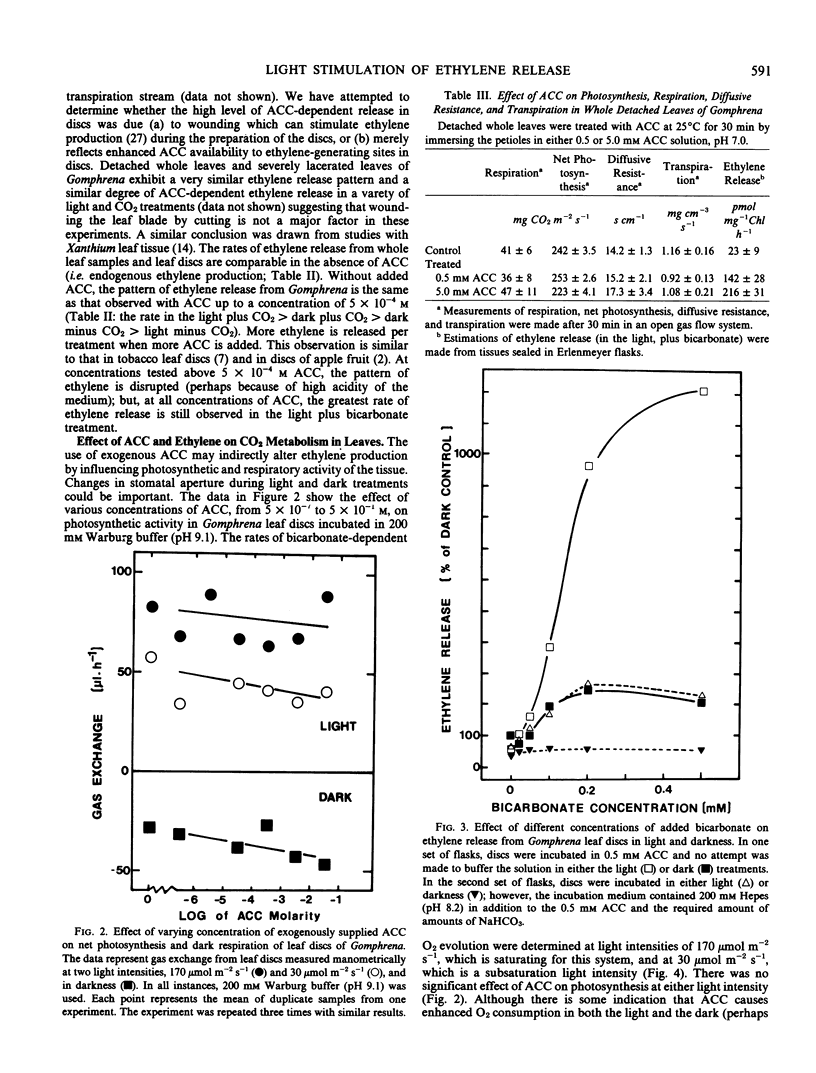
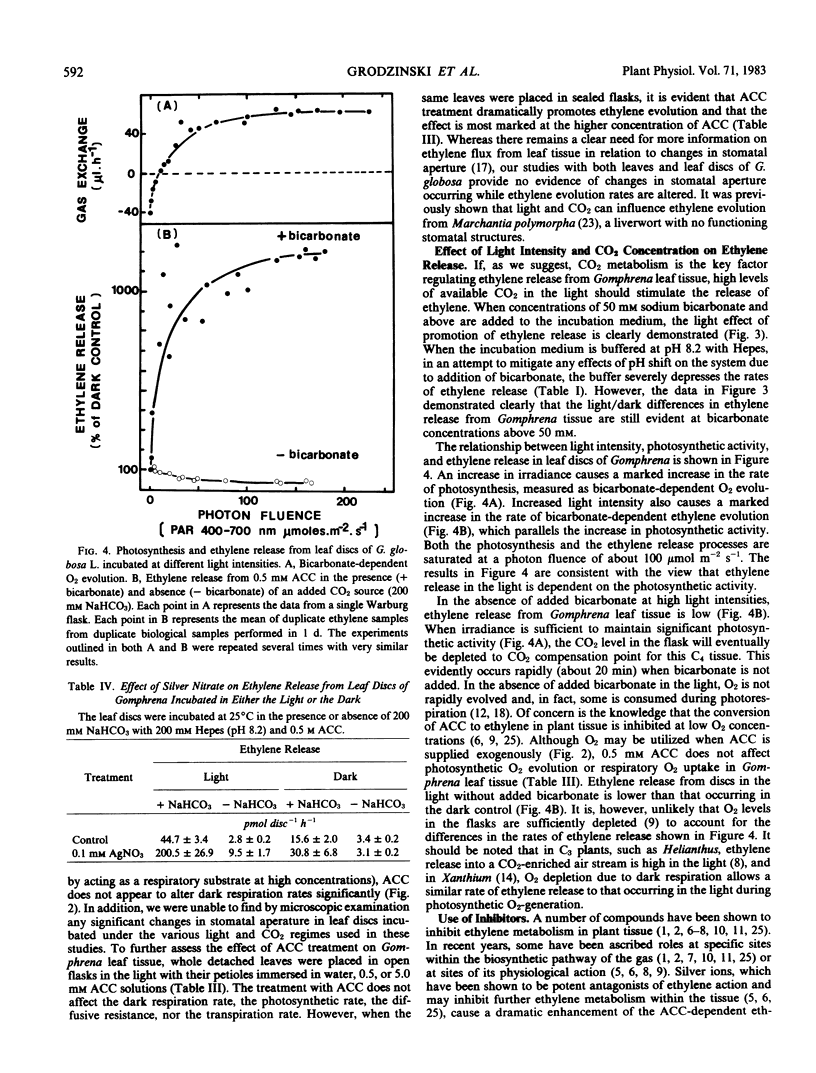
Leaf tissue in the light with a source of CO2 sufficient to maintain photosynthesis always generates 3 to 4 times more ethylene than tissue in the dark. Conversely, the lowest rate of ethylene release occurs when leaf tissue is illuminated and photosynthetic activity depletes the CO2 to the compensation point. Ethylene release in the dark is also stimulated by CO2 either added to the flask as bicarbonate or generated by dark respiration. Ethylene release increases dramatically and in parallel with photosynthesis at increasing light intensities in this C4 plant. Ethylene release appears dependent on CO2 both in the light and in the dark. Therefore, it is suggested that the important factor regulating the evolution of ethylene gas from leaves of Gomphrena may be CO2 metabolism rather than light per se.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Burgoon A. C., Anderson J. D., Solomos T., Lieberman M. Some Characteristics of the System Converting 1-Aminocyclopropane-1-carboxylic Acid to Ethylene. Plant Physiol. 1981 Jan;67(1):80–84. doi: 10.1104/pp.67.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. Effect of carbon dioxide and light on ethylene production in intact sunflower plants. Plant Physiol. 1982 May;69(5):1222–1225. doi: 10.1104/pp.69.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan K. R., Bassi P. K., Spencer M. S. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol. 1981 Oct;68(4):831–834. doi: 10.1104/pp.68.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Raschke K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiol. 1981 Jul;68(1):33–40. doi: 10.1104/pp.68.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H. H., Badger M. R., Watson L. Variations in Kinetic Properties of Ribulose-1,5-bisphosphate Carboxylases among Plants. Plant Physiol. 1981 Jun;67(6):1151–1155. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]


