Abstract
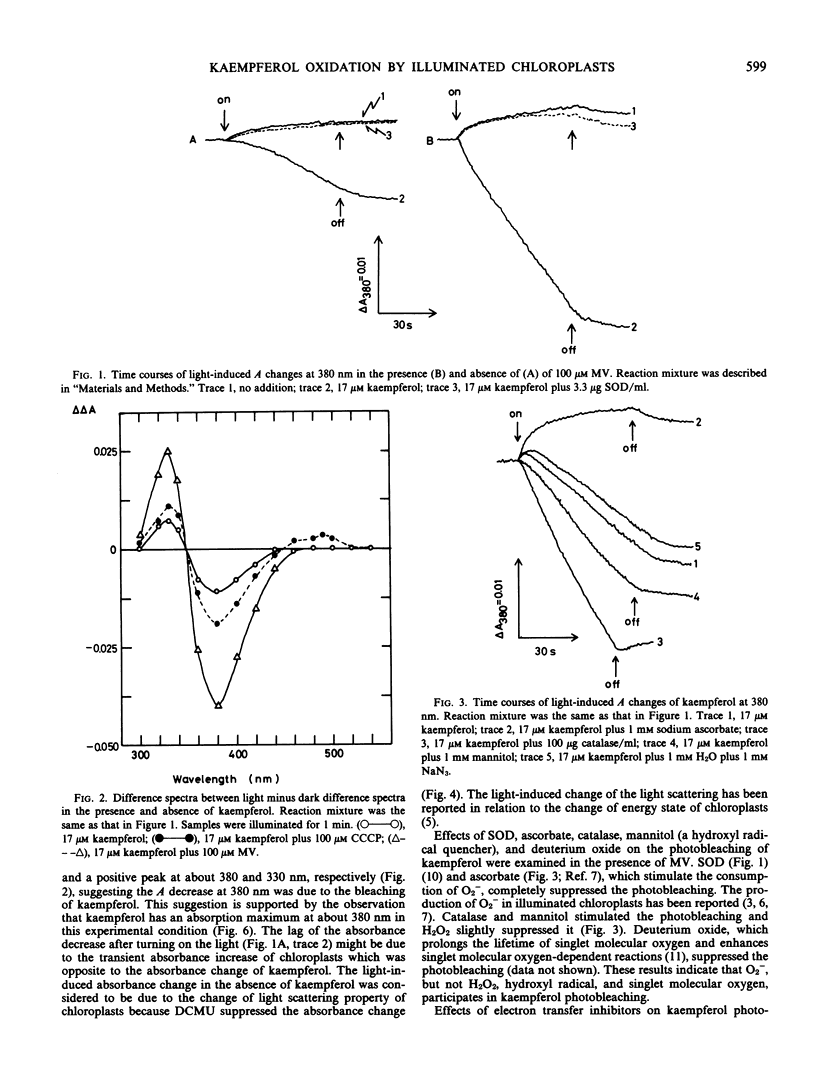
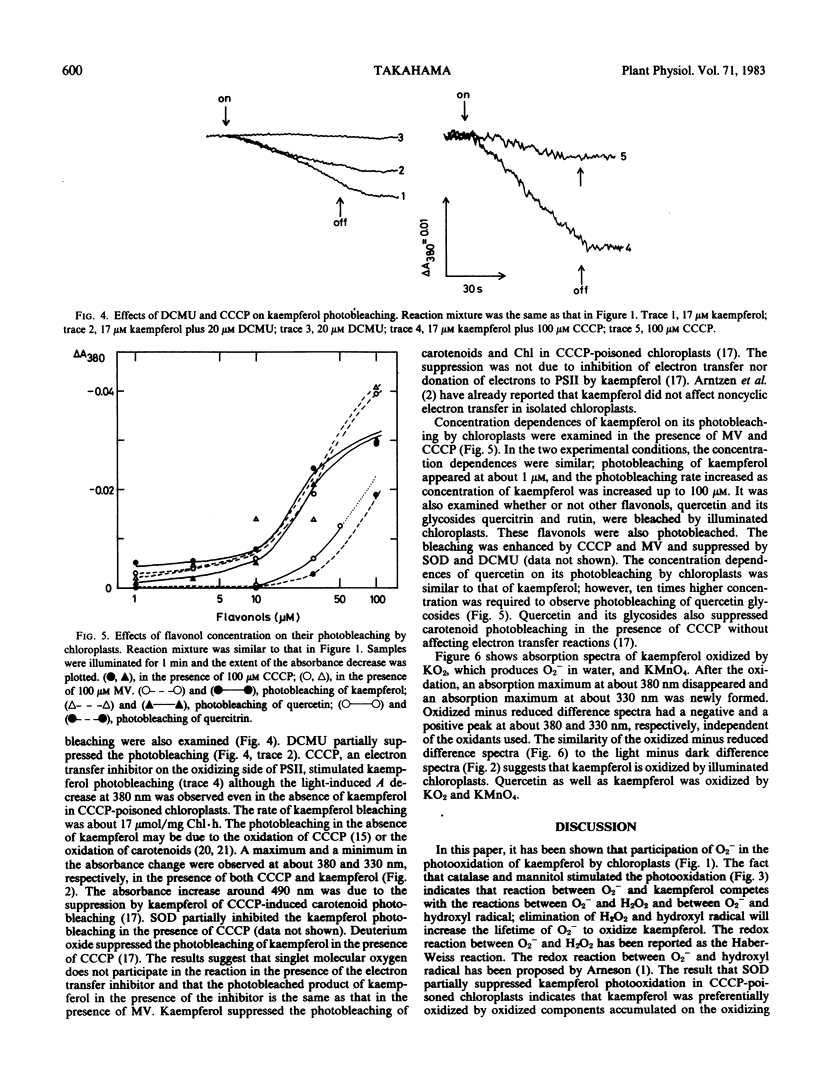
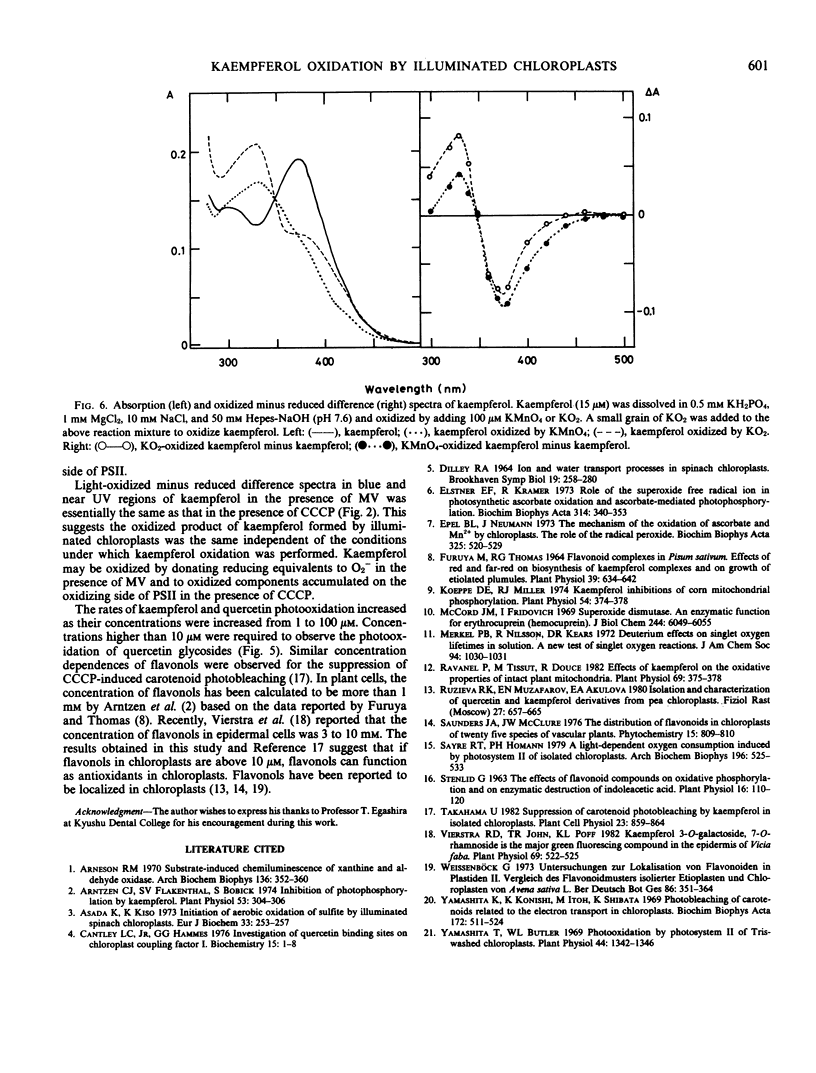
Bleaching of kaempferol by illuminated chloroplasts was observed at 380 nanometers. The photobleaching was stimulated by methyl viologen and suppressed by superoxide dismutase indicating the participation of O2− in the reaction. An electron transfer inhibitor on the oxidizing side of photosystem II, carbonylcyanide m-chlorophenylhydrazone (CCCP), stimulated the photobleaching and 3-(3,4-dichlorophenyl)-1,1-dimethylurea partially suppressed it. The stimulation by CCCP suggests that kaempferol is also bleached on the oxidizing side of photosystem II. The spectrum of kaempferol bleaching in the presence of methyl viologen was the same as that in the presence of CCCP having a maximum in absorbance decrease at around 380 nanometers. When kaempferol was oxidized by KMnO2 or KO2, the oxidized minus reduced difference spectra had also a negative peak at about 380 nanometers. The results suggest that kaempferol was oxidized by illuminated chloroplasts.
The rate of kaempferol photooxidation increased as its concentration was increased from 1 to 100 micromolar. The rate of quercetin photooxidation also increased as its concentration was increased from 1 to 100 micromolar. Concentration of quercetin glycosides higher than 10 micromolar was required to detect their photobleaching by illuminated chloroplasts. From these results, it is postulated that flavonols function as antioxidants in chloroplasts.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneson R. M. Substrate-induced chemiluminescence of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1970 Feb;136(2):352–360. doi: 10.1016/0003-9861(70)90205-5. [DOI] [PubMed] [Google Scholar]
- Arntzen C. J., Falkenthal S. V., Bobick S. Inhibition of photophosphorylation by kaempferol. Plant Physiol. 1974 Feb;53(2):304–306. doi: 10.1104/pp.53.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kiso K. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem. 1973 Mar 1;33(2):253–257. doi: 10.1111/j.1432-1033.1973.tb02677.x. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Kramer R. Role of the superoxide free radical ion in photosynthetic ascorbate oxidation and ascorbate-mediated photophosphorylation. Biochim Biophys Acta. 1973 Sep 26;314(3):340–353. doi: 10.1016/0005-2728(73)90118-7. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Neumann J. The mechanism of the oxidation of ascorbate and MN2+ by chloroplasts. The role of the radical superoxide. Biochim Biophys Acta. 1973 Dec 14;325(3):520–529. doi: 10.1016/0005-2728(73)90211-9. [DOI] [PubMed] [Google Scholar]
- Furuya M., Thomas R. G. Flavonoid Complexes in Pisum sativum. II. Effects of Red and Far-Red Light on Biosynthesis of Kaempferol Complexes and on Growth in Etiolated Plumules. Plant Physiol. 1964 Jul;39(4):634–642. doi: 10.1104/pp.39.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe D. E., Miller R. J. Kaempferol inhibitions of corn mitochondrial phosphorylation. Plant Physiol. 1974 Sep;54(3):374–378. doi: 10.1104/pp.54.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ravanel P., Tissut M., Douce R. Effects of kaempferol on the oxidative properties of intact plant mitochondria. Plant Physiol. 1982 Feb;69(2):375–378. doi: 10.1104/pp.69.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre R. T., Homann P. H. A light-dependent oxygen consumption induced by photosystem II of isolated chloroplasts. Arch Biochem Biophys. 1979 Sep;196(2):525–533. doi: 10.1016/0003-9861(79)90304-7. [DOI] [PubMed] [Google Scholar]
- Vierstra R. D., John T. R., Poff K. L. Kaempferol 3-O-Galactoside, 7-O-Rhamnoside is the Major Green Fluorescing Compound in the Epidermis of Vicia faba. Plant Physiol. 1982 Feb;69(2):522–525. doi: 10.1104/pp.69.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Konishi K., Itoh M., Shibata K. Photo-bleaching of carotenoids related to the electron transport in chloroplasts. Biochim Biophys Acta. 1969 Apr 8;172(3):511–524. doi: 10.1016/0005-2728(69)90147-9. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photooxidation by photosystem II of tris-washed chloroplasts. Plant Physiol. 1969 Sep;44(9):1342–1346. doi: 10.1104/pp.44.9.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]


