Abstract
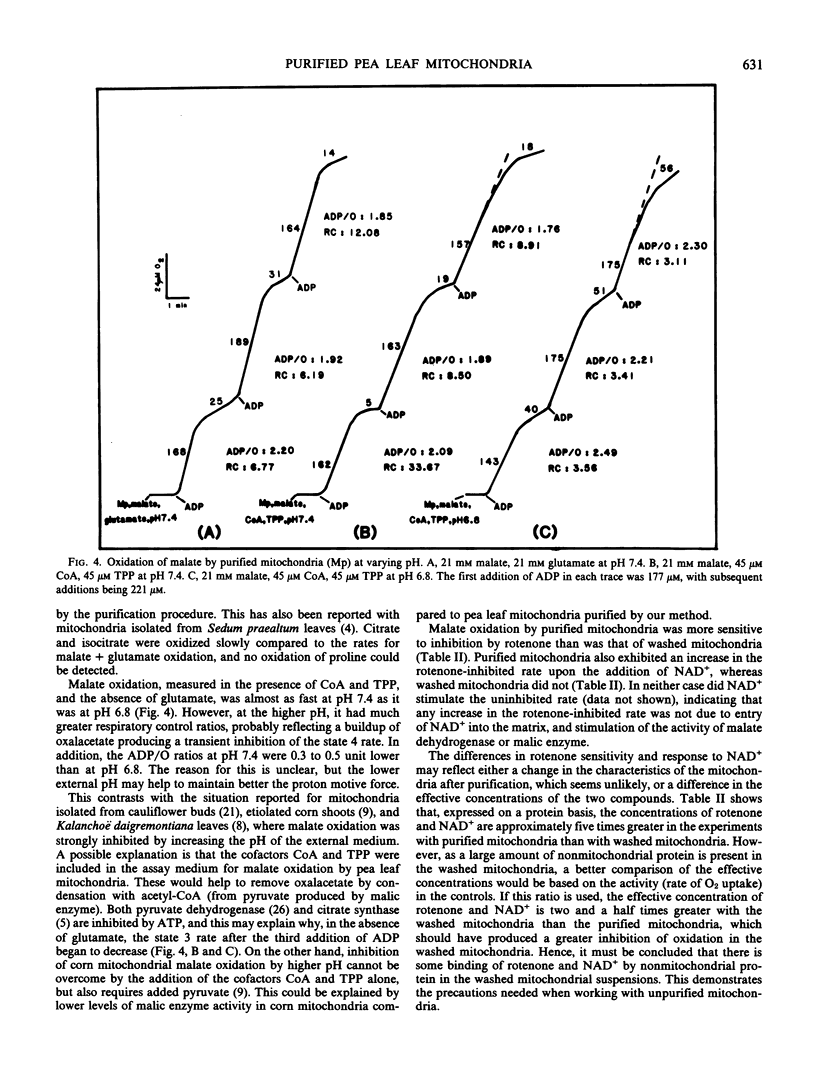
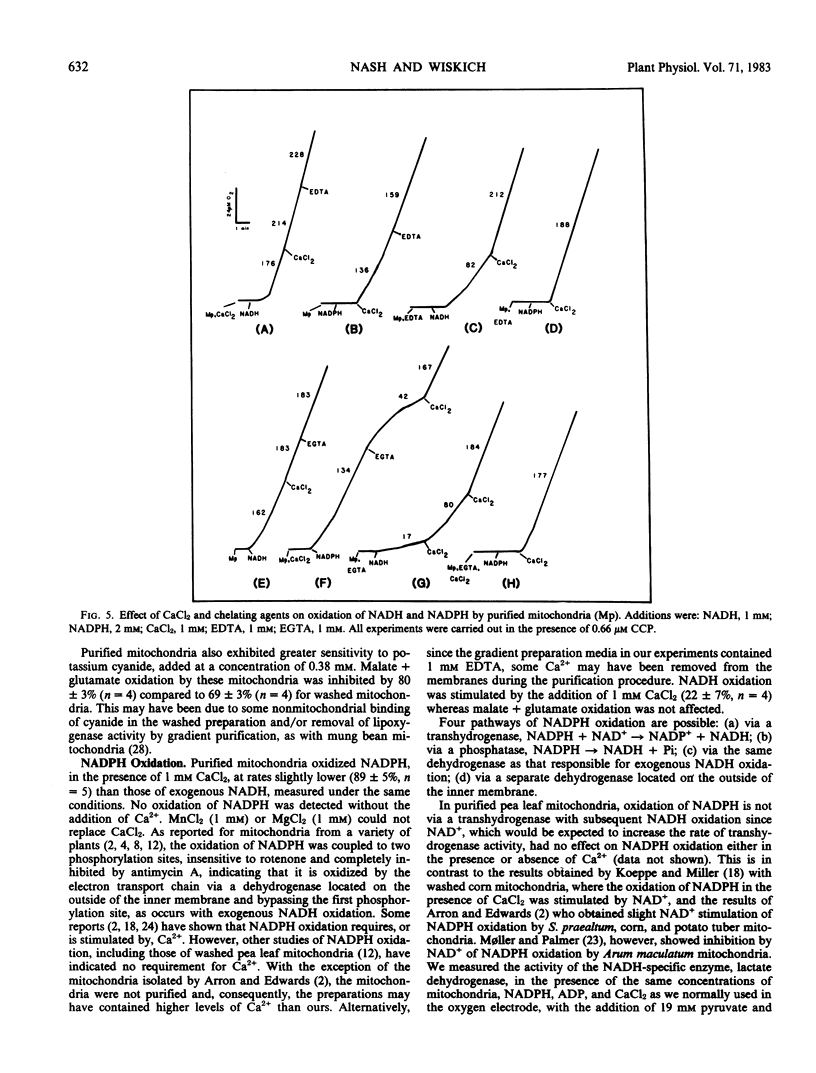
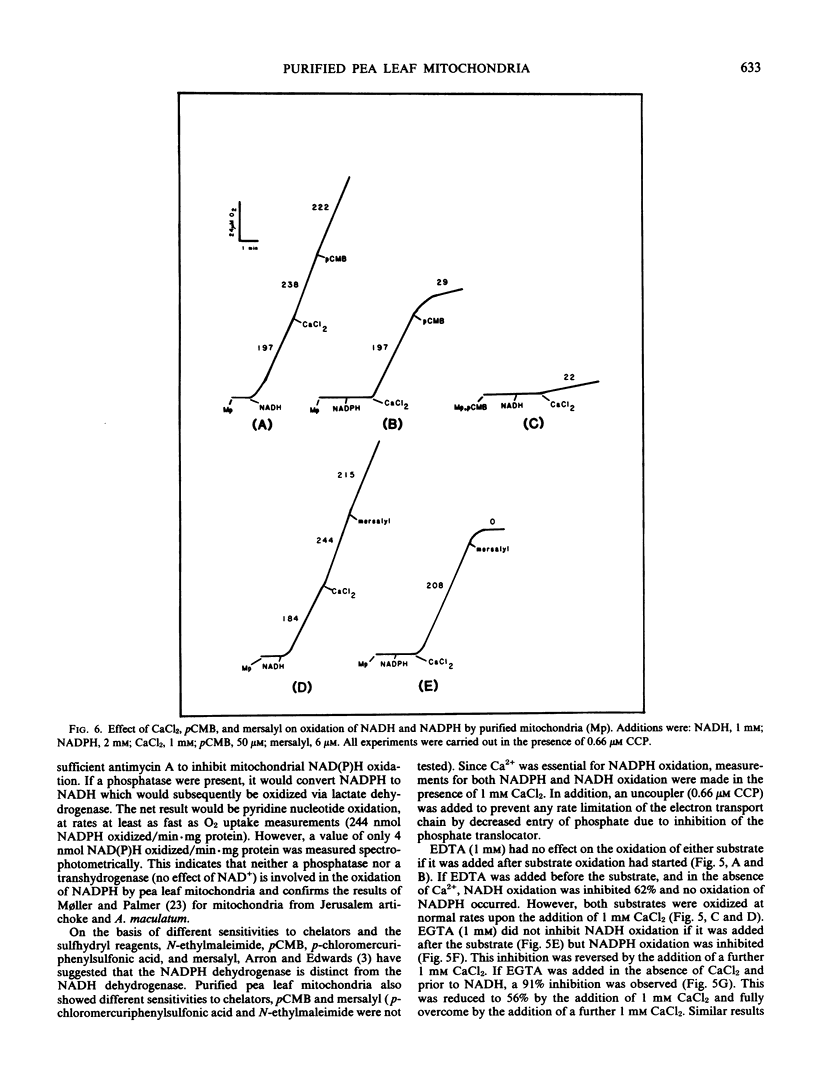
Mitochondria isolated from pea leaves (Pisum sativum L. var Massey Gem) and purified on a linear sucrose density gradient were substantially free of contamination by Chl and peroxisomes. They showed high respiratory rates and good respiratory control and ADP/O ratios. Malate, glutamate, succinate, glycine, pyruvate, α-ketoglutarate, NADH, and NADPH were oxidized but little or no oxidation of citrate, isocitrate, or proline was detected. The oxidation of NADPH by the purified mitochondria did not occur via a transhydrogenase or phosphatase converting it to NADH. NADPH oxidation had an absolute requirement for added Ca2+, whereas NADH oxidation proceeded in its absence. In addition, oxidation of the two substrates showed different sensitivities to chelators and sulfhydryl reagents, and faster rates of O2 uptake were observed with both substrates than with either alone. This indicates that the NADPH dehydrogenase is distinct from the exogenous NADH dehydrogenase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron G. P., Edwards G. E. Oxidation of Reduced Nicotinamide Adenine Dinucleotide Phosphate by Potato Mitochondria: INHIBITION BY SULFHYDRYL REAGENTS. Plant Physiol. 1980 Apr;65(4):591–594. doi: 10.1104/pp.65.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron G. P., Edwards G. E. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by plant mitochondria. Can J Biochem. 1979 Dec;57(12):1392–1399. doi: 10.1139/o79-185. [DOI] [PubMed] [Google Scholar]
- Arron G. P., Spalding M. H., Edwards G. E. Isolation and Oxidative Properties of Intact Mitochondria from the Leaves of Sedum praealtum: A Crassulacean Acid Metabolism Plant. Plant Physiol. 1979 Aug;64(2):182–186. doi: 10.1104/pp.64.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbareschi D., Longo G. P., Servettaz O., Zulian T., Longo C. P. Citrate synthetase in mitochondria and glyoxysomes of maize scutellum. Plant Physiol. 1974 Jun;53(6):802–807. doi: 10.1104/pp.53.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A., Gardeström P., Ericson I. Method to Obtain a Chlorophyll-free Preparation of Intact Mitochondria from Spinach Leaves. Plant Physiol. 1980 Sep;66(3):442–445. doi: 10.1104/pp.66.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977 Apr;59(4):630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A. Malate Decarboxylation by Kalanchoë daigremontiana Mitochondria and Its Role in Crassulacean Acid Metabolism. Plant Physiol. 1980 Apr;65(4):675–679. doi: 10.1104/pp.65.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Isolation and properties of the outer membrane of plant mitochondria. Arch Biochem Biophys. 1975 Nov;171(1):117–123. doi: 10.1016/0003-9861(75)90014-4. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Haas R., Wrage K., Heinz E. Phospholipid composition of chlorophyll-free mitochondria isolated via protoplasts from oat mesophyll cells. Hoppe Seylers Z Physiol Chem. 1981 Aug;362(8):1069–1078. doi: 10.1515/bchm2.1981.362.2.1069. [DOI] [PubMed] [Google Scholar]
- Jackson C., Dench J. E., Hall D. O., Moore A. L. Separation of mitochondria from contaminating subcellular structures utilizing silica sol gradient centrifugation. Plant Physiol. 1979 Jul;64(1):150–153. doi: 10.1104/pp.64.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe D. E., Miller R. J. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by isolated corn mitochondria. Plant Physiol. 1972 Mar;49(3):353–357. doi: 10.1104/pp.49.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Møller I. M., Johnston S. P., Palmer J. M. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981 Feb 15;194(2):487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Rubin P. M. Plant Pyruvate Dehydrogenase Complex: II. ATP-Dependent Inactivation and Phosphorylation. Plant Physiol. 1977 Jan;59(1):1–3. doi: 10.1104/pp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- Siedow J. N., Girvin M. E. Alternative Respiratory Pathway: ITS ROLE IN SEED RESPIRATION AND ITS INHIBITION BY PROPYL GALLATE. Plant Physiol. 1980 Apr;65(4):669–674. doi: 10.1104/pp.65.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]