Abstract
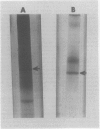
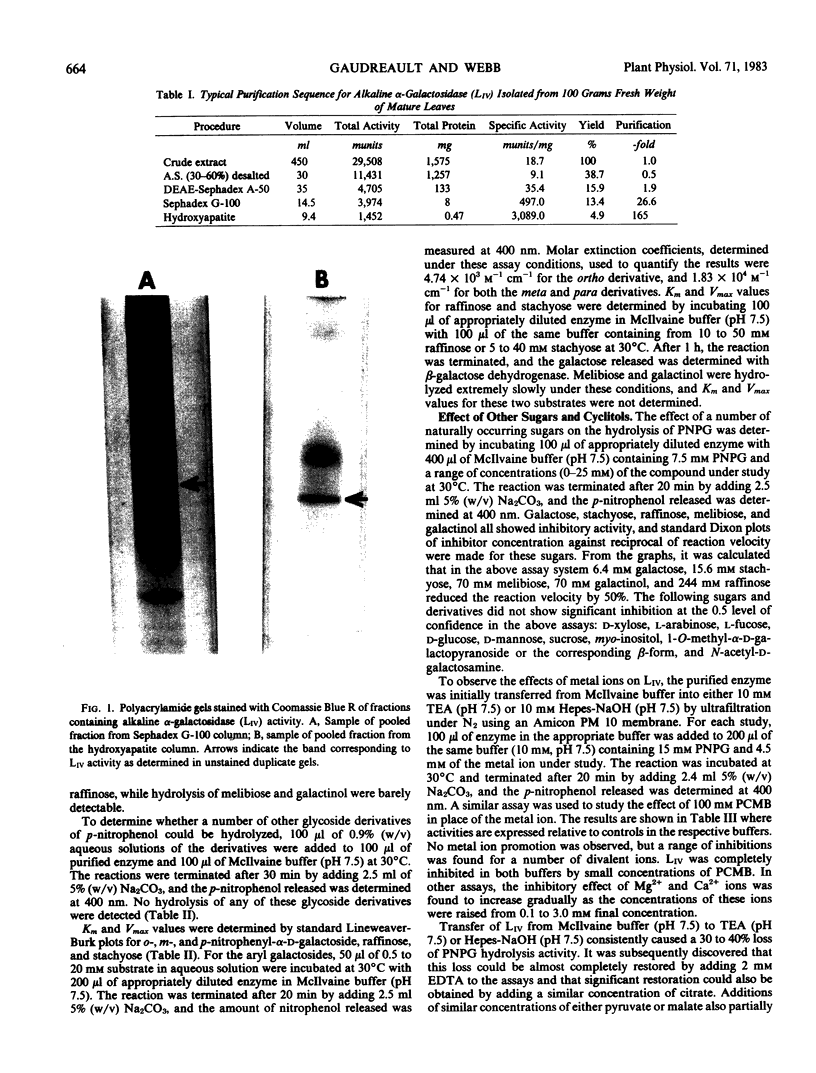
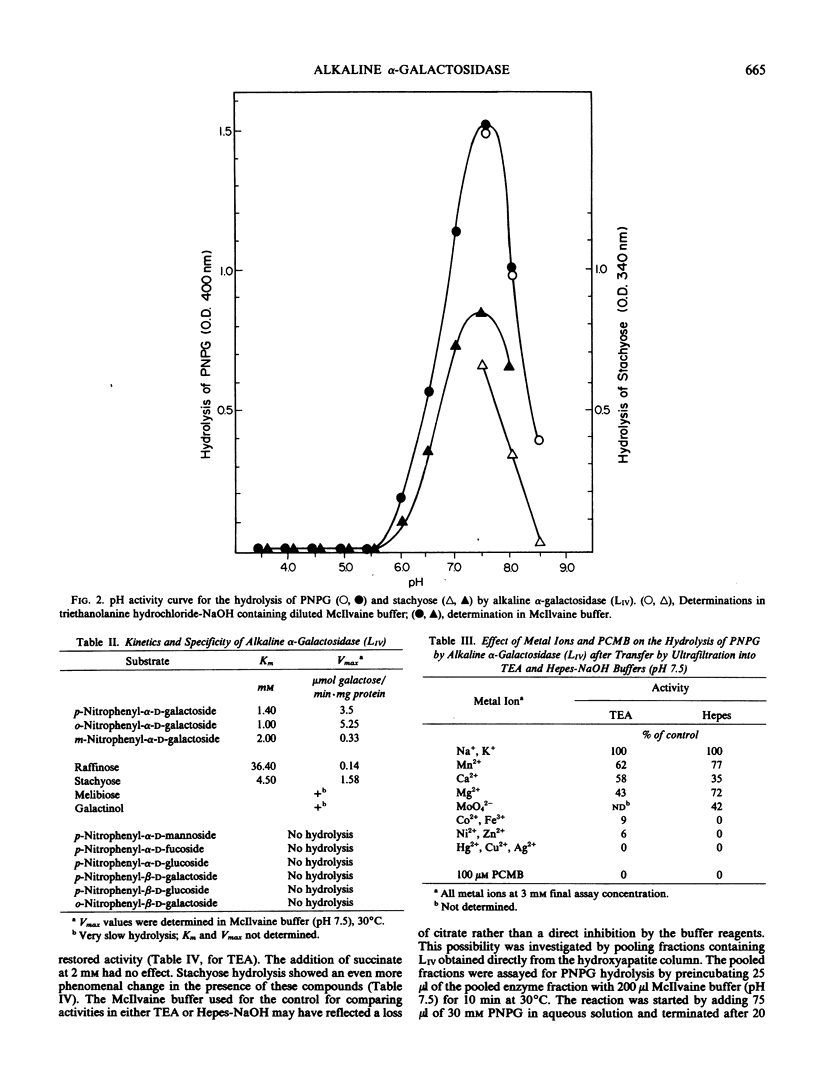
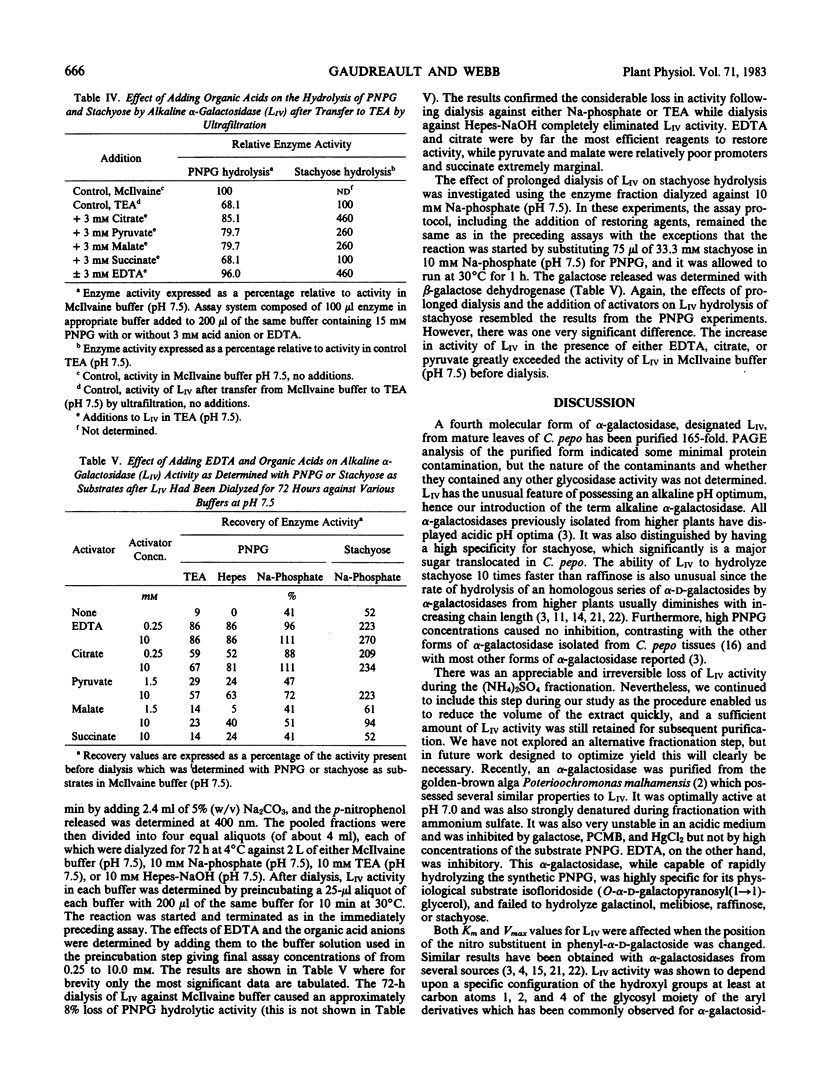
A fourth molecular from of α-galactosidase, designated LIV, an alkaline α-galactosidase, was isolated from leaves of Cucurbita pepo and purified 165-fold. It was active over a narrow pH range with optimal hydrolysis of p-nitrophenyl-α-d-galactoside and stachyose at pH 7.5. The rate of stachyose hydrolysis was 10 times that of raffinose. Km determinations in McIlvaine buffer (200 millimolar Na2-phosphate, 100 millimolar citric acid, pH 7.5) for p-nitrophenyl-α-d-galactoside, stachyose, and raffinose were 1.40, 4.5, and 36.4 millimolar, respectively. LIV was partially inhibited by Ca2+, Mg2+, and Mn2+, more so by Ni2+, Zn2+, and Co2+, and highly so by Cu2+, Ag2+, Hg2+ and by p-chloromercuribenzoate. It was not inhibited by high concentrations of the substrate p-nitrophenyl-α-d-galactoside or by myo-inositol, but α-d-galactose was a strong inhibitor. As observed for most other forms of α-galactosidase, LIV only catalyzed the hydrolysis of glycosides possessing the α-d-galactose configuration at C1, C2, and C4, and did not hydrolyze p-nitrophenyl-α-d-fucoside (α-d-galactose substituted at C6). The enzyme was highly sensitive to buffers and chelating agents. Maximum hydrolytic activity for p-nitrophenyl-α-d-galactoside was obtained in McIlvaine buffer (pH 7.5). In 10 millimolar triethanolaminehydrochloride-NaOH (pH 7.5) or 10 millimolar Hepes-NaOH (pH 7.5), hydrolytic activity was virtually eliminated, but the addition of low concentrations of either ethylenediaminetetraacetate or citrate to these buffers restored activity almost completely. Partial restoration of activity was also observed, but at higher concentrations, with pyruvate and malate. Similar effects were found for stachyose hydrolysis, but in addition some inhibition of LIV in McIlvaine buffer, possibly due to the high phosphate concentration, was observed with this substrate. It is questionable whether the organic acid anions possess any regulatory control of LIVin vivo. It was possible that the results reflected the ability of these anions, and ethylene-diaminetetraacetate, to restore LIV activity through coordination with some toxic cation introduced as a buffer contaminant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- FRENCH D. The raffinose family of oligosaccharides. Adv Carbohydr Chem. 1954;9:149–184. doi: 10.1016/s0096-5332(08)60375-6. [DOI] [PubMed] [Google Scholar]
- Gatt S., Baker E. A. Purification and separation of alpha- and beta-galactosidases from spinach leaves. Biochim Biophys Acta. 1970 Apr 22;206(1):125–135. doi: 10.1016/0005-2744(70)90089-6. [DOI] [PubMed] [Google Scholar]
- Petek F., Villarroya E., Courtois J. E. Purification et propriétés de l'alpha-galactosidase des graines germées de Vicia sativa. Eur J Biochem. 1969 Apr;8(3):395–402. doi: 10.1111/j.1432-1033.1969.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Sharma C. B. Selective inhibition of -galactosidases by myoinositol. Biochem Biophys Res Commun. 1971 May 7;43(3):572–579. doi: 10.1016/0006-291x(71)90652-8. [DOI] [PubMed] [Google Scholar]
- Smart E. L., Pharr D. M. Characterization of alpha-Galactosidase from Cucumber Leaves. Plant Physiol. 1980 Oct;66(4):731–734. doi: 10.1104/pp.66.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Li S. C., Li Y. T. Alpha-galactosidase from Mortierella vinacea. Crystallization and properties. J Biol Chem. 1970 Feb 25;245(4):781–786. [PubMed] [Google Scholar]
- Tornheim K., Gilbert T. R., Lowenstein J. M. Metal contaminants in commercial preparations of nucleotides. Anal Biochem. 1980 Mar 15;103(1):87–93. doi: 10.1016/0003-2697(80)90241-9. [DOI] [PubMed] [Google Scholar]
- Womack F. C., Colowick S. P. Proton-dependent inhibition of yeast and brain hexokinases by aluminum in ATP preparations. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5080–5084. doi: 10.1073/pnas.76.10.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]