Abstract
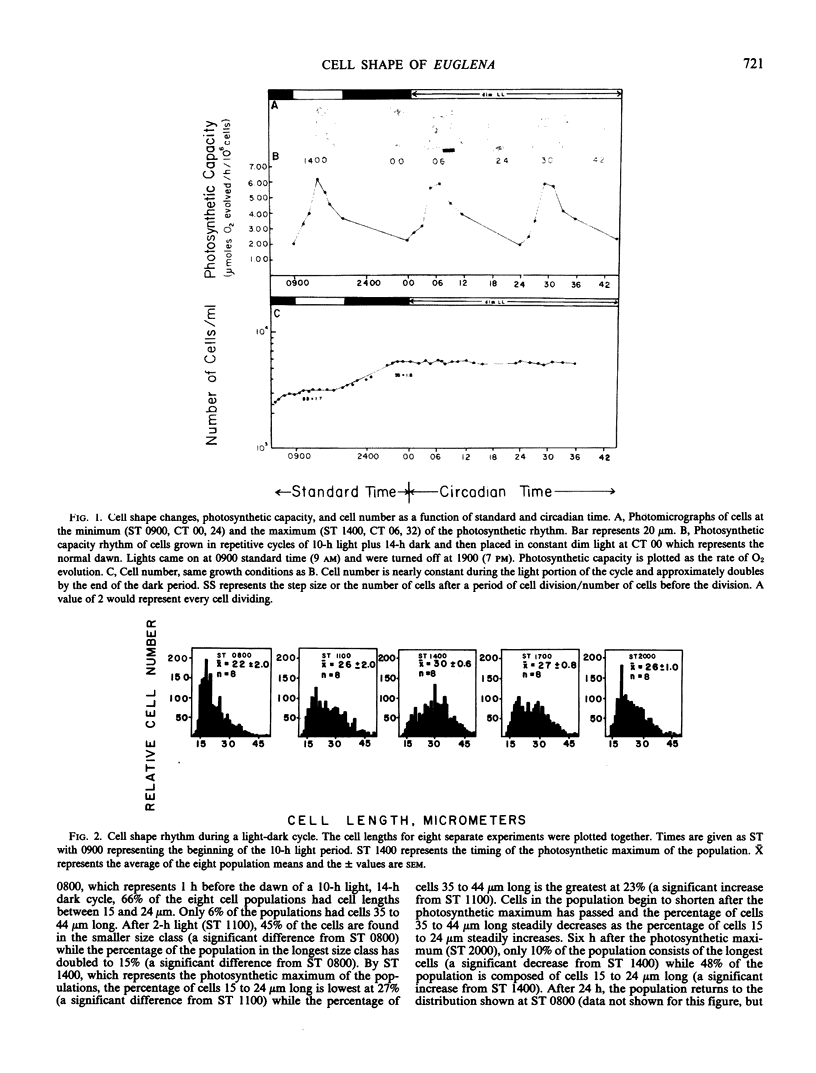
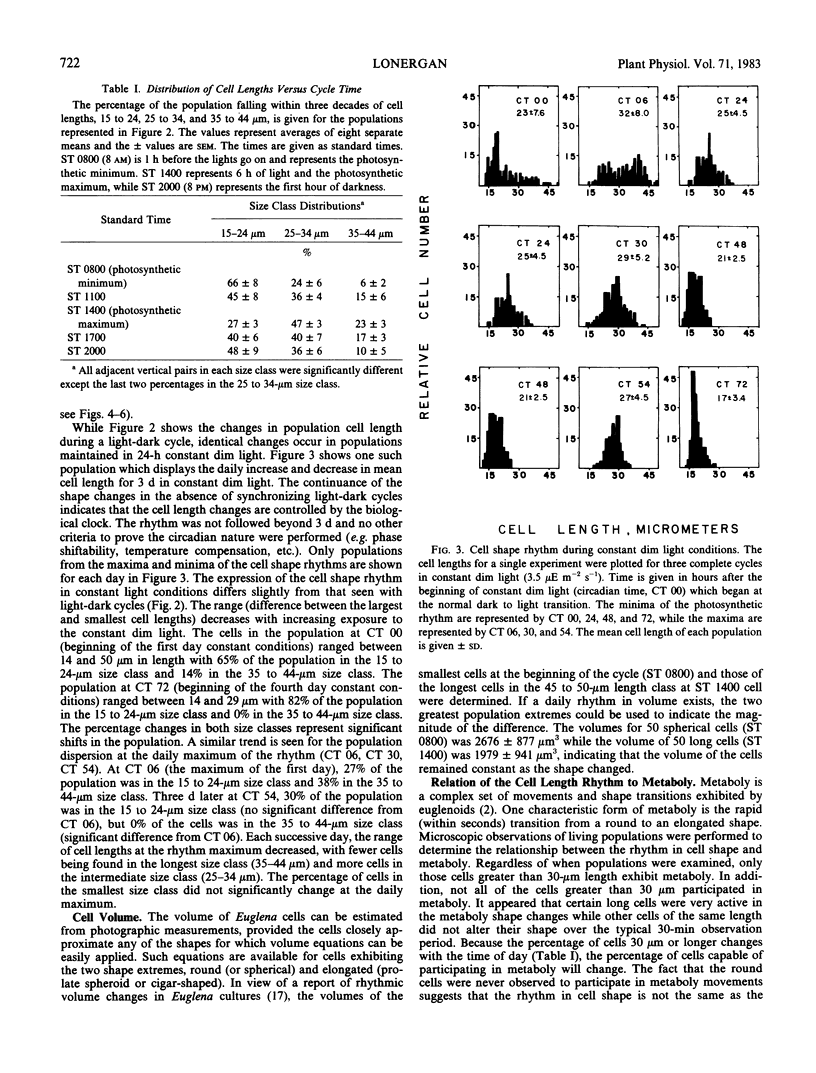
The alga Euglena gracilis Z. changes its shape two times per day when grown under the synchronizing effect of a daily light-dark cycle. At the beginning of the light period when photosynthetic capacity is low, the population of cells is largely spherical in shape. The mean cell length of the population increases to a maximum in the middle of the light period when photosynthetic capacity is greatest, and then decreases for the remainder of the 24-hour period. The population becomes spherical by the end of the 24-hour period when the cycle reinitiates. These changes are also observed under constant dim light conditions (up to 72 hours) and are therefore controlled by the biological clock and represent a circadian rhythm in cell shape. In constant dim light, the cell division rhythm is either arrested or slowed considerably, while the cell shape rhythm continues.
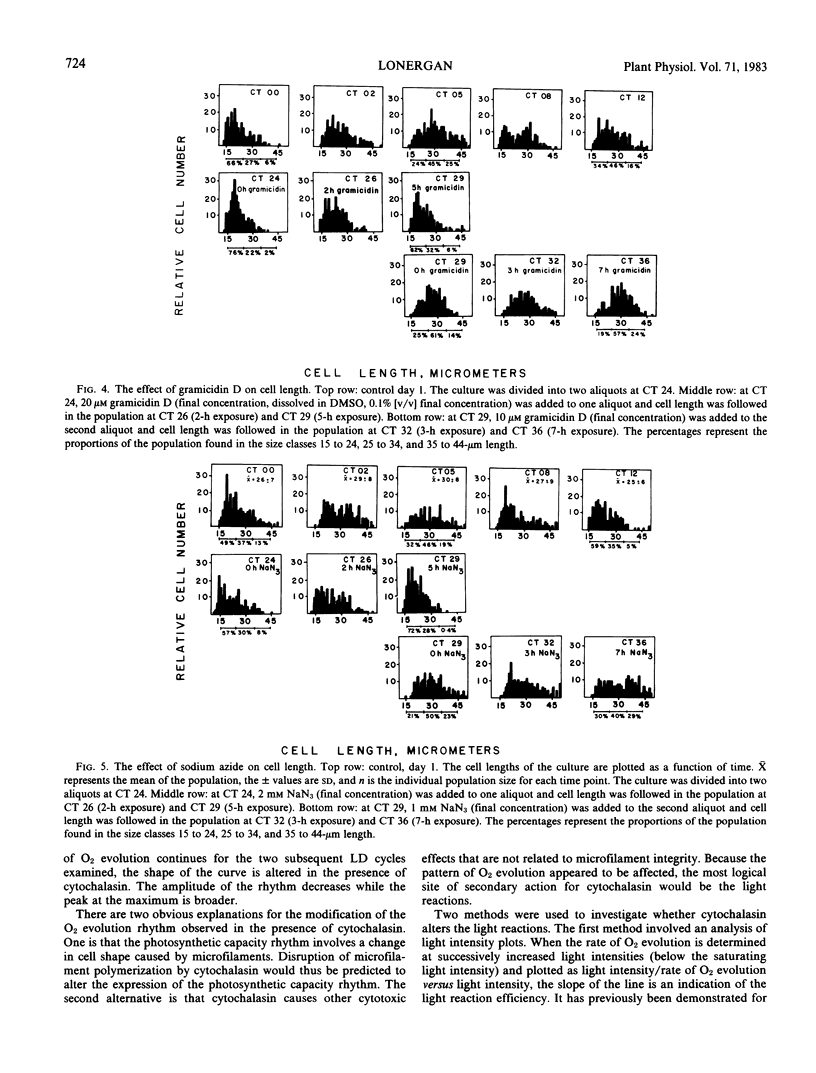
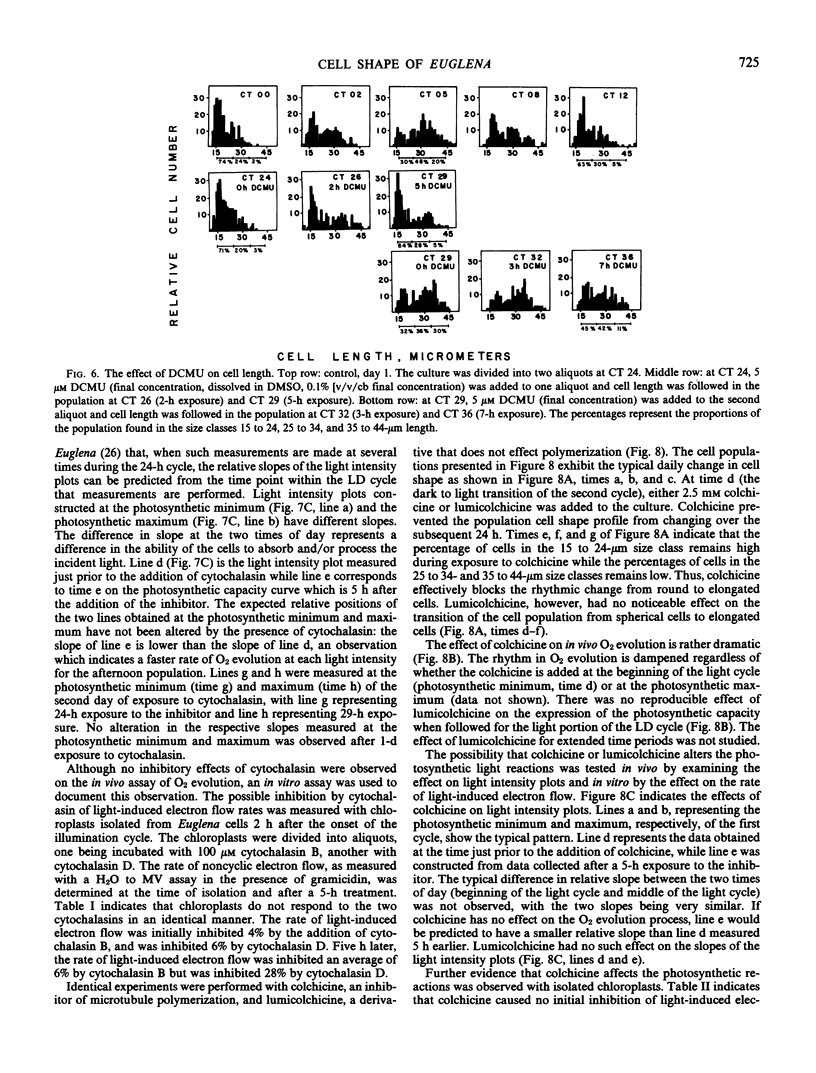
The involvement of respiratory and photosynthetic pathways in the cell shape changes was investigated with energy pathway inhibitors. Antimycin A and NaN3 both inhibited the round to long and long to round shape changes, indicating that the respiratory pathways are involved. DCMU and atrazine inhibited the round to long shape change but did not affect the long to round transition, indicating that light-induced electron flow is necessary only for the round to long shape change.
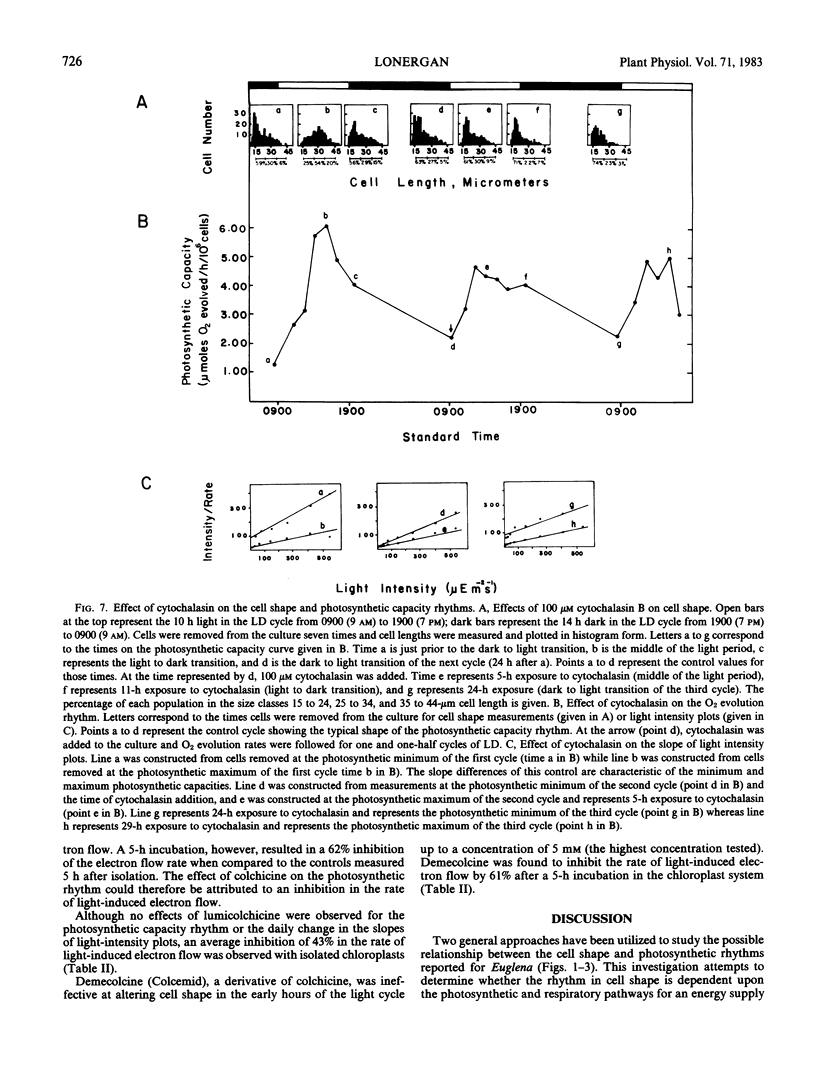
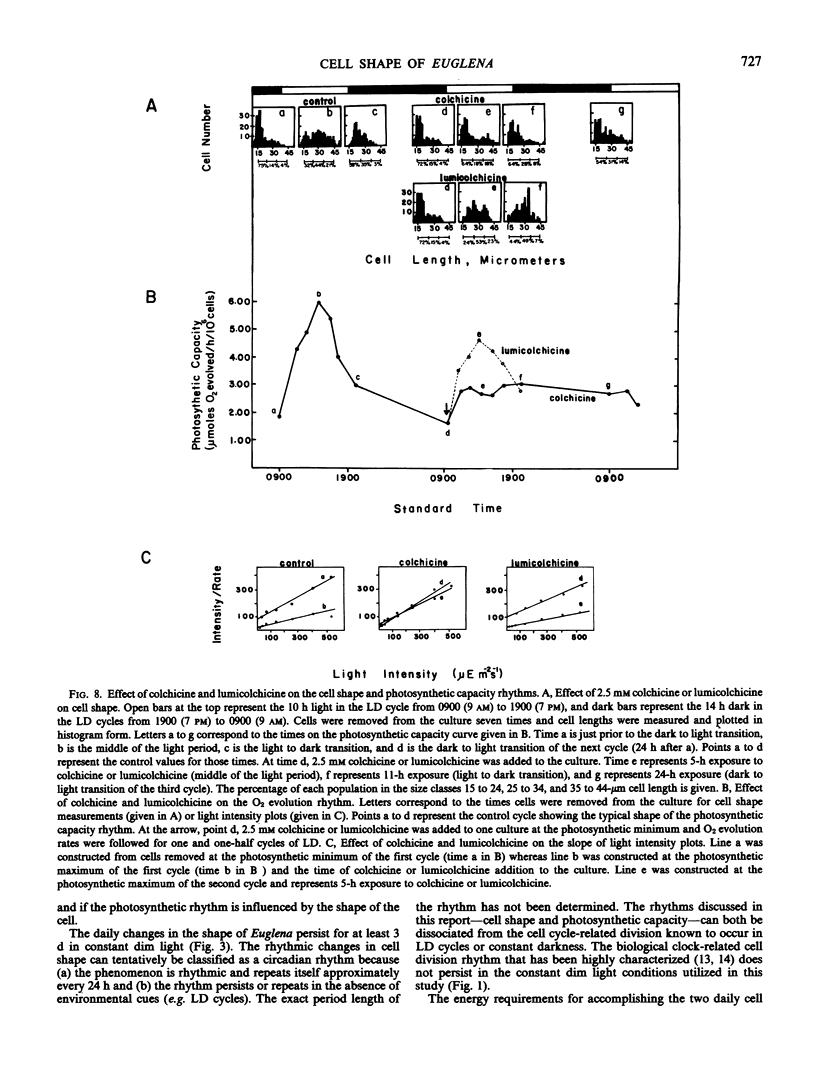
The influence of the cell shape changes on the photosynthetic reactions was investigated by altering cell shape with the cytoskeletal inhibitors cytochalasin and colchicine. Both inhibitors blocked the round to long and long to round shape changes. Cytochalasin B was found to have minimal cytotoxic effects on the photosynthetic reactions, but colchicine significantly inhibited light-induced electron flow and the in vivo expression of the photosynthetic rhythm.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck G. B., Brown D. L. Microtubule biogenesis and cell shape in Ochromonas. I. The distribution of cytoplasmic and mitotic microtubules. J Cell Biol. 1973 Feb;56(2):340–359. doi: 10.1083/jcb.56.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O. Microfilaments and cytoplasmic streaming: inhibition of streaming with cytochalasin. J Cell Sci. 1973 Jan;12(1):327–343. doi: 10.1242/jcs.12.1.327. [DOI] [PubMed] [Google Scholar]
- Britz S. J., Briggs W. R. Circadian rhythms of chloroplast orientation and photosynthetic capacity in ulva. Plant Physiol. 1976 Jul;58(1):22–27. doi: 10.1104/pp.58.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Martins S. A. Circadian rhythms in Neurospora crassa: effects of unsaturated fatty acids. J Bacteriol. 1979 Feb;137(2):912–915. doi: 10.1128/jb.137.2.912-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L., Bouck G. B. Microtubule biogenesis and cell shape in Ochromonas. II. The role of nucleating sites in shape development. J Cell Biol. 1973 Feb;56(2):360–378. doi: 10.1083/jcb.56.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds L. N., Jr Studies on synchronously dividing cultures of Euglena gracilis Klebs (strain Z). II. Patterns of biosynthesis during the cell cycle. J Cell Physiol. 1965 Oct;66(2):159–181. doi: 10.1002/jcp.1030660205. [DOI] [PubMed] [Google Scholar]
- Halaban R. Glucose transport-deficient mutant of Neurospora crassa with an unusual rhythmic growth pattern. J Bacteriol. 1975 Mar;121(3):1056–1063. doi: 10.1128/jb.121.3.1056-1063.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Bouck G. B. Immunological and structural evidence for patterned intussusceptive surface growth in a unicellular organism. A postulated role for submembranous proteins and microtubules. J Cell Biol. 1976 Jun;69(3):693–715. doi: 10.1083/jcb.69.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Lin D. C., Spudich J. A., Kun E. Inhibition of mitochondrial contraction by cytochalasin B. FEBS Lett. 1973 Dec 1;37(2):241–243. doi: 10.1016/0014-5793(73)80469-7. [DOI] [PubMed] [Google Scholar]
- Lonergan T. A. A circadian rhythm in the rate of light-induced electron flow in three leguminous species. Plant Physiol. 1981 Nov;68(5):1041–1046. doi: 10.1104/pp.68.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T. A., Sargent M. L. Regulation of the Photosynthesis Rhythm in Euglena gracilis: I. Carbonic Anhydrase and Glyceraldehyde-3-Phosphate Dehydrogenase Do Not Regulate the Photosynthesis Rhythm. Plant Physiol. 1978 Feb;61(2):150–153. doi: 10.1104/pp.61.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T. A., Sargent M. L. Regulation of the Photosynthesis Rhythm in Euglena gracilis: II. Involvement of Electron Flow through Both Photosystems. Plant Physiol. 1979 Jul;64(1):99–103. doi: 10.1104/pp.64.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern D., Brody S. Circadian rhythms in Neurospora crassa: effects of saturated fatty acids. J Bacteriol. 1979 Sep;139(3):977–983. doi: 10.1128/jb.139.3.977-983.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel M., Roth C., Fink J., Fyfe G., Lacy P. Effects of cytochalasins B and D on alloxan inhibition of insulin release. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1089–1096. doi: 10.1016/0006-291x(75)90469-6. [DOI] [PubMed] [Google Scholar]
- Wagner G., Haupt W., Laux A. Reversible inhibition of chloroplast movement by cytochalasin B in the green alga mougeofia. Science. 1972 May 19;176(4036):808–809. doi: 10.1126/science.176.4036.808. [DOI] [PubMed] [Google Scholar]



