Abstract
Cotyledons of light-grown soybean (Glycine max L. var Wayne) seedlings were used as a model system to study the possibility that aging requires qualitative changes in protein synthesis. Cotyledons reached a final stage of senescence and then abscised about 22 days after imbibition. Cotyledon senescence was reversed at 20 days after germination by epicotyl removal. Thereafter, the cotyledons regained much of the chlorophyll, RNA, protein, and polyribosomes lost during aging.
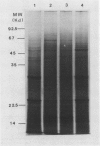
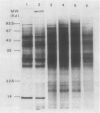
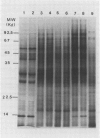
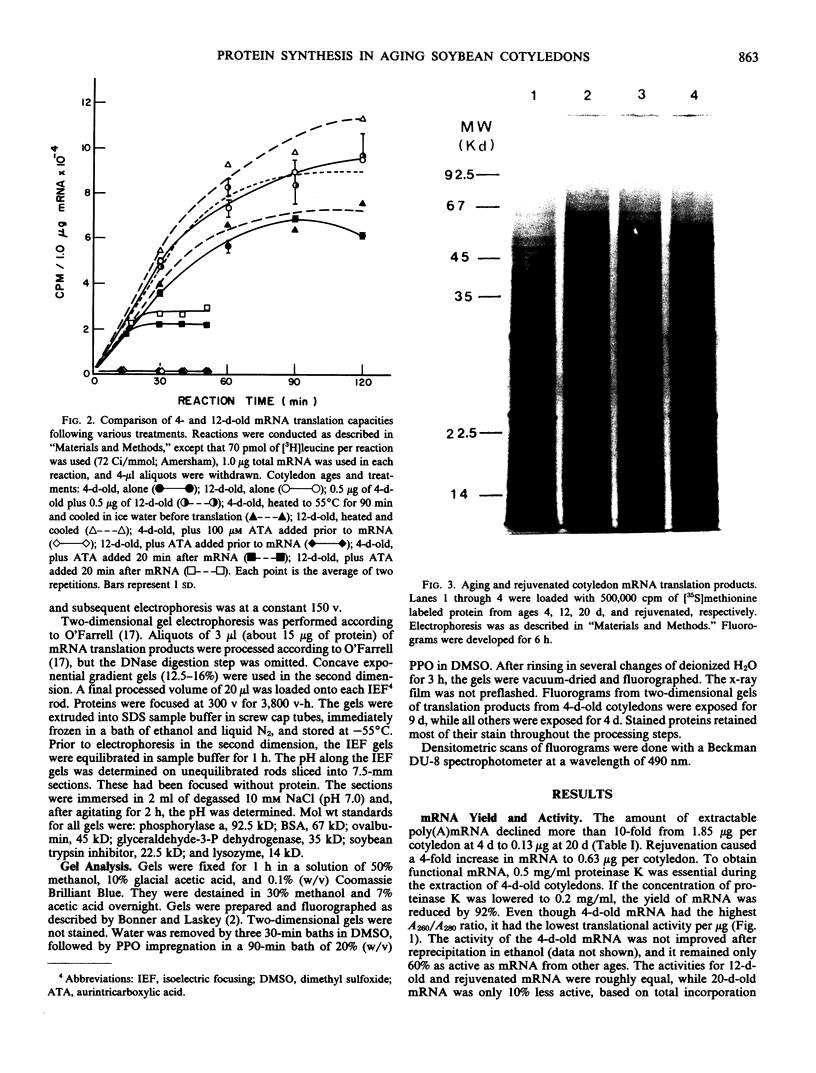
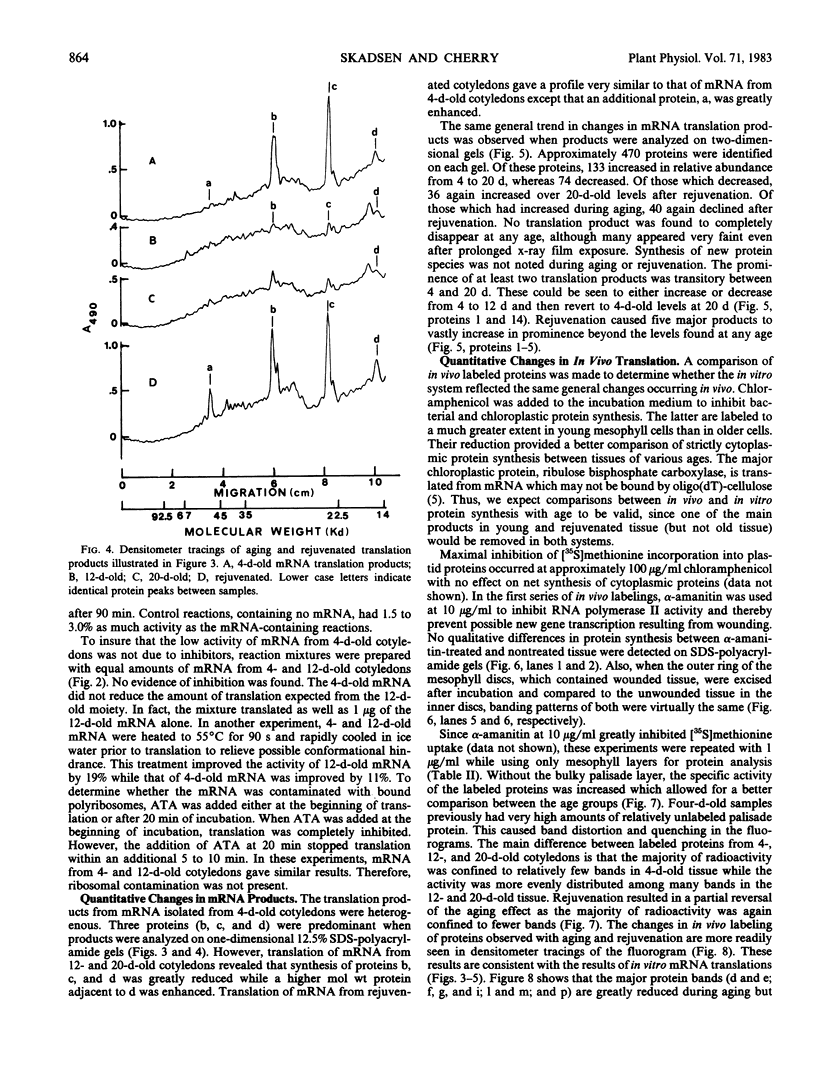
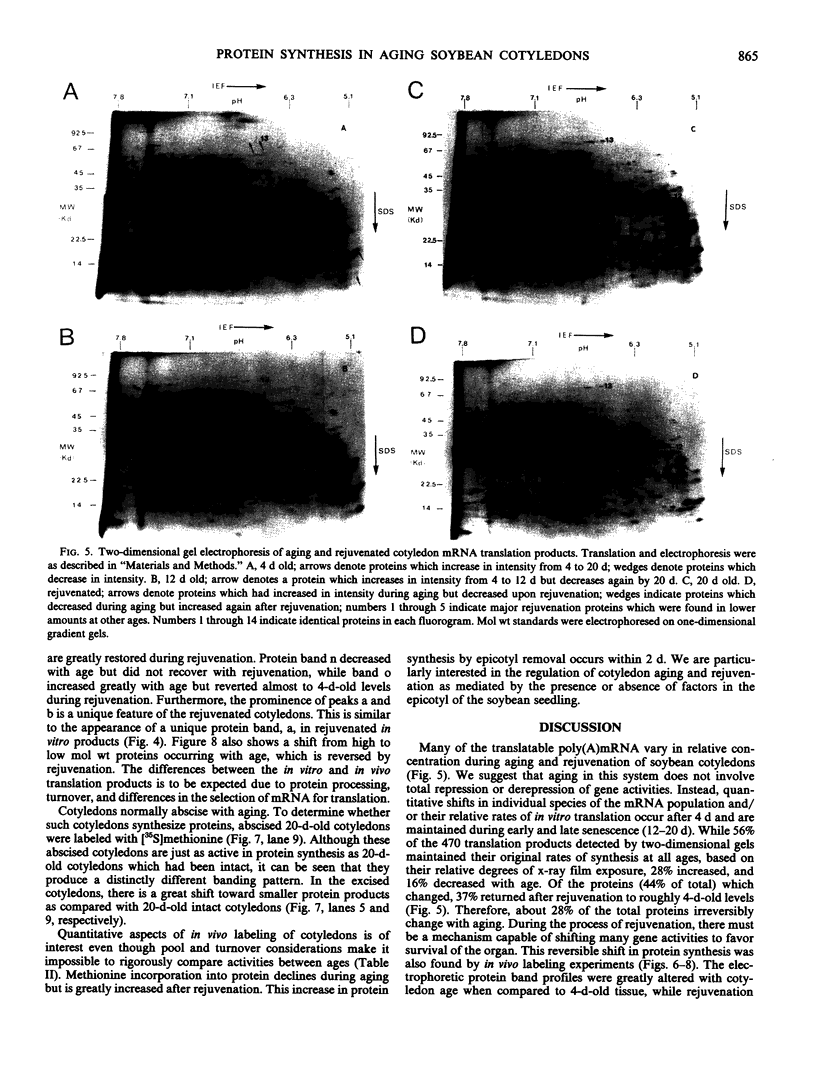
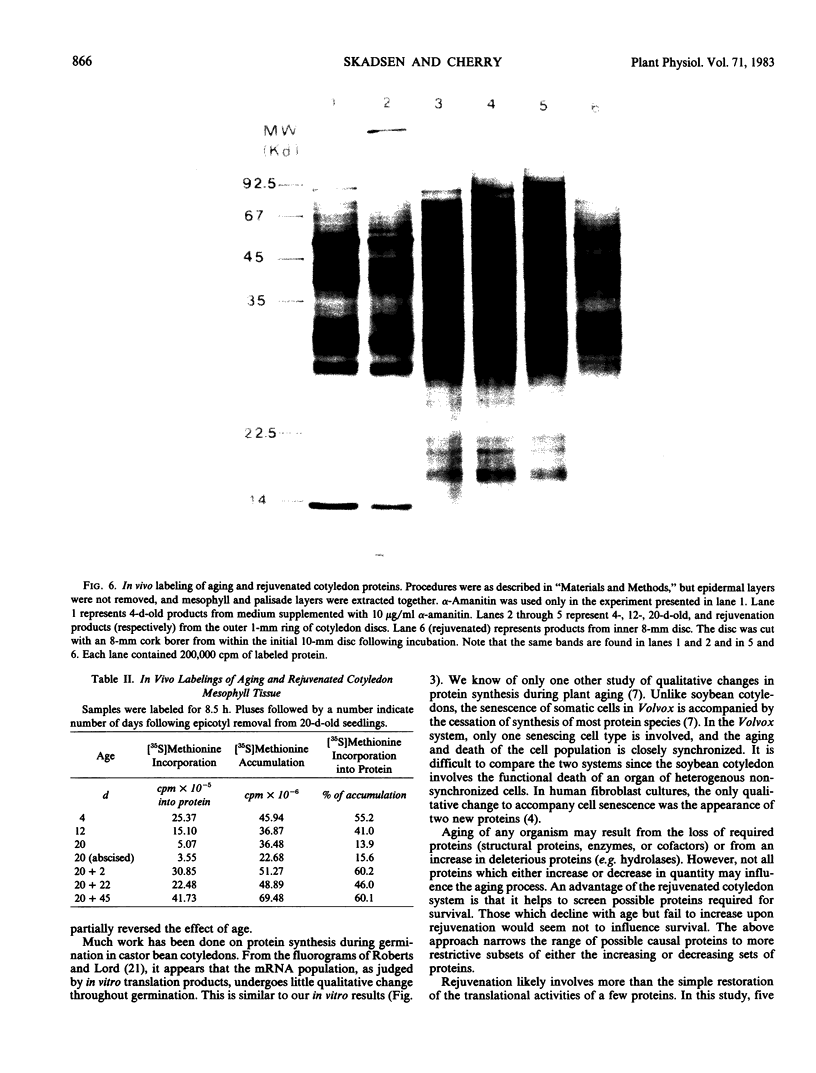
Total poly(A)mRNA was extracted from 4-, 12-, 20-day-old, and rejuvenated cotyledons and translated in a wheat germ system. Comparison of translation products on two-dimensional O'Farrell gels showed that many translation products increased in quantity during aging, while roughly half as many decreased. Rejuvenation returned the translation products to approximately 4-day-old levels in roughly half of those products which were diminished with age. Conversely, almost one-third of the products which had increased with age decreased with rejuvenation. None of the translation products were totally lost nor were newly synthesized products detected during aging. Therefore, aging in this system probably does not involve complete gene repression or depression. The observation that epicotyl removal causes a reversal in the levels of various proteins synthesized in vitro was corroborated by similar observations following in vivo labeling of cotyledon sections and analysis by SDS-polyacrylamide gel electrophoresis and fluorography. Densitometric scans of fluorograms revealed a gradual shift in profiles of both in vitro and in vivo translation products during aging. Rejuvenated cotyledon proteins had a profile resembling that of 4-day-old cotyledons. The overall level of [35S]methionine incorporation into protein in vivo declined gradually during aging but was restored to 4-day-old levels within 2 days after epicotyl removal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt D. L., Lee G. T., Moley J. F. Patterns of peptide synthesis in senescent and presenescent human fibroblasts. J Cell Physiol. 1979 Jan;98(1):193–198. doi: 10.1002/jcp.1040980121. [DOI] [PubMed] [Google Scholar]
- Gelvin S., Howell S. H. Identification and Precipitation of the Polyribosomes in Chlamydomonas reinhardi Involved in the Synthesis of the Large Subunit of d-ribulose-1,5-bisphosphate Carboxylase. Plant Physiol. 1977 Mar;59(3):471–477. doi: 10.1104/pp.59.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hagen G., Kochert G. Protein synthesis in a new system for the study of senescence. Exp Cell Res. 1980 Jun;127(2):451–457. doi: 10.1016/0014-4827(80)90452-8. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Ma Y., Buchbinder B. U., Pyne J. W., Sun S. M., Bliss F. A. Messenger RNA for G1 protein of French bean seeds: Cell-free translation and product characterization. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul W. R. Nucleic Acid and protein metabolism of senescing and regenerating soybean cotyledons. Plant Physiol. 1974 Jul;54(1):36–40. doi: 10.1104/pp.54.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Pendergrass W. R., Sprague C. A., Martin G. M. Dominance of the senescent phenotype in heterokaryons between replicative and post-replicative human fibroblast-like cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2231–2235. doi: 10.1073/pnas.71.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborne D. J. Effect of Kinetin on Protein & Nucleic Acid Metabolism in Xanthium Leaves During Senescence. Plant Physiol. 1962 Sep;37(5):595–602. doi: 10.1104/pp.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Pollard J. W., Friesen J. D., Stanners C. P. Stuttering: high-level mistranslation in animal and bacterial cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1091–1095. doi: 10.1073/pnas.75.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. Developmental changes in the activity of messenger RNA isolated from germinating castor bean endosperm. Plant Physiol. 1979 Oct;64(4):630–634. doi: 10.1104/pp.64.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler B., Hirsch G., Gusseck D., Johnson R., Bick M. Codon-restriction theory by aging and development. J Theor Biol. 1971 Dec;33(3):429–474. doi: 10.1016/0022-5193(71)90091-9. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Induced senescence of intact wheat seedlings and its reversibility. Plant Physiol. 1977 Jun;59(6):1039–1042. doi: 10.1104/pp.59.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]