Abstract
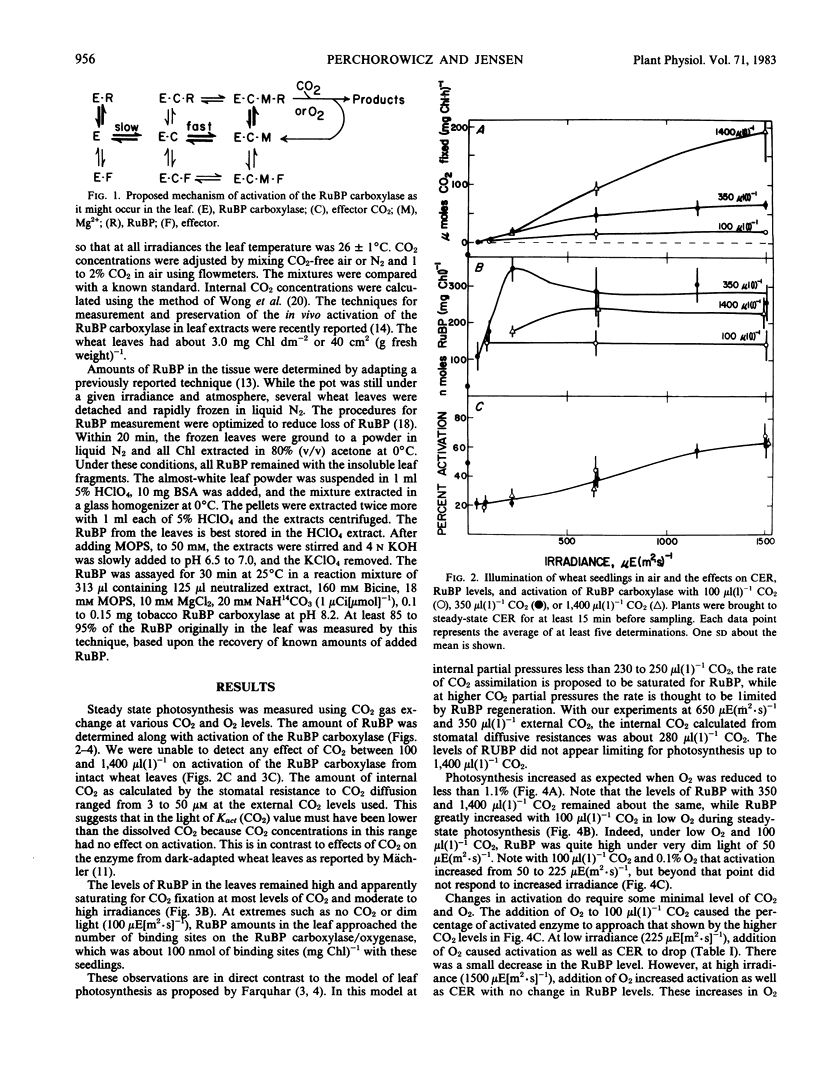
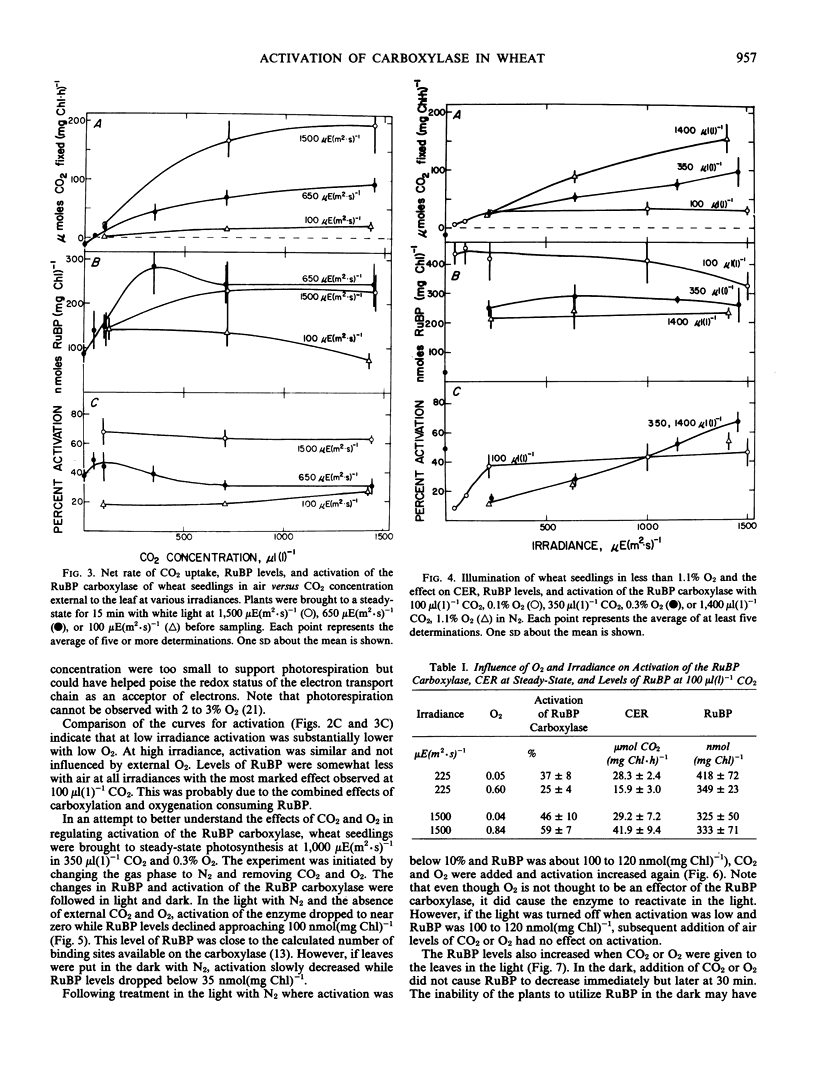
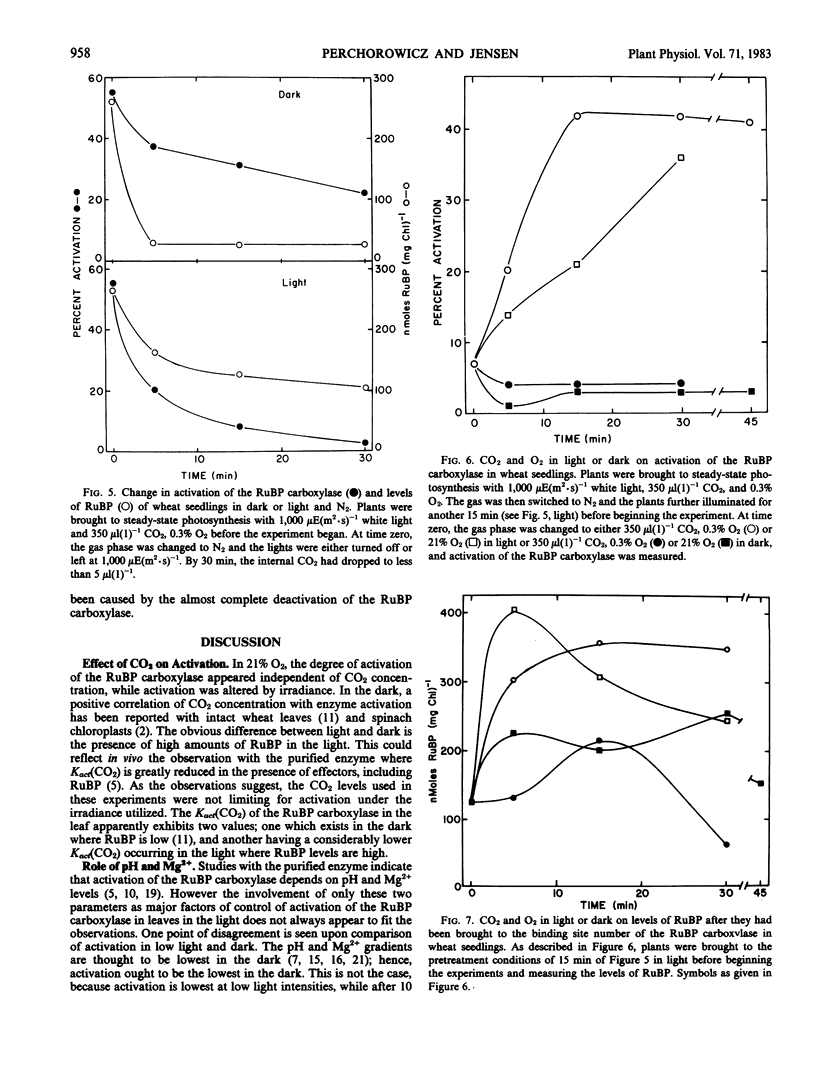
Photosynthetic carbon assimilation in plants is regulated by activity of the ribulose 1,5-bisphosphate (RuBP) carboxylase/oxygenase. Although the carboxylase requires CO2 to activate the enzyme, changes in CO2 between 100 and 1,400 microliters per liter did not cause changes in activation of the leaf carboxylase in light. With these CO2 levels and 21% O2 or 1% or less O2, the levels of ribulose bisphosphate were high and not limiting for CO2 fixation. With high leaf ribulose bisphosphate, the Kact(CO2) of the carboxylase must be lower than in dark, where RuBP is quite low in leaves. When leaves were illuminated in the absence of CO2 and O2, activation of the carboxylase dropped to zero while RuBP levels approached the binding site concentration of the carboxylase, probably by forming the inactive enzyme-RuBP complex.
The mechanism for changing activation of the RuBP carboxylase in the light involves not only Mg2+ and pH changes in the chloroplast stroma, but also the effects of binding RuBP to the enzyme. In light when RuBP is greater than the binding site concentration of the carboxylase, Mg2+ and pH most likely determine the ratio of inactive enzyme-RuBP to active enzyme-CO2-Mg2+-RuBP forms. Higher irradiances favor more optimal Mg2+ and pH, with greater activation of the carboxylase and increased photosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Lorimer G. H. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981 Apr 14;20(8):2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Activation of ribulose bisphosphate carboxylase in intact chloroplasts by CO2 and light. Arch Biochem Biophys. 1978 Jan 15;185(1):39–48. doi: 10.1016/0003-9861(78)90141-8. [DOI] [PubMed] [Google Scholar]
- Hatch A. L., Jensen R. G. Regulation of ribulose-1,5-bisphosphate carboxylase from tobacco: changes in pH response and affinity for CO2 and Mg2+ induced by chloroplast intermediates. Arch Biochem Biophys. 1980 Dec;205(2):587–594. doi: 10.1016/0003-9861(80)90142-3. [DOI] [PubMed] [Google Scholar]
- Krause G. H. The high-energy state of the thylakoid system as indicated by chlorophyll fluorescence and chloroplast shrinkage. Biochim Biophys Acta. 1973 Apr 5;292(3):715–728. doi: 10.1016/0005-2728(73)90019-4. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- McCurry S. D., Pierce J., Tolbert N. E., Orme-Johnson W. H. On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J Biol Chem. 1981 Jul 10;256(13):6623–6628. [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Measurement and preservation of the in vivo activation of ribulose 1,5-bisphosphate carboxylase in leaf extracts. Plant Physiol. 1982 May;69(5):1165–1168. doi: 10.1104/pp.69.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R. Evidence of a Low Stromal Mg Concentration in Intact Chloroplasts in the Dark: I. STUDIES WITH THE IONOPHORE A23187. Plant Physiol. 1981 May;67(5):985–989. doi: 10.1104/pp.67.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Bahr J. T., Jensen R. G. Measurement of ribulose 1,5-bisphosphate from spinach chloroplasts. Plant Physiol. 1979 Nov;64(5):876–879. doi: 10.1104/pp.64.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishnick M., Lane M. D., Scrutton M. C. The interaction of metal ions with ribulose 1,5-diphosphate carboxylase from spinach. J Biol Chem. 1970 Oct 10;245(19):4939–4947. [PubMed] [Google Scholar]


