Graphical abstract
Keywords: Ultrasound-assisted extraction, Ficus carica polysaccharides, Structural characterization, Antioxidant properties, Hypoglycemic effects, Immunomodulatory activities
Highlights
-
•
Ultrasound-assisted extraction of Ficus carica polysaccharides (FCPS) was optimized by RSM.
-
•
FCPS was characterized as pyran-type polysaccharides with a molecular weight of 4.224 × 104 Da.
-
•
FCPS exhibited remarkable antioxidant activities and alleviated oxidative stress damage.
-
•
FCPS promoted insulin resistance in hepatocytes and activated the immune response.
Abstract
In this research, the ultrasound-assisted extraction (UAE) conditions of the water-soluble polysaccharide (FCPS) from Ficus carica fruits were optimized using the response surface methodology. The optimal FCPS yield was 7.97 % achieved by conducting ultrasound-assisted extraction four times at a solid–liquid ratio of 1:20 (g/mL) and an ultrasound temperature of 70 °C. Then, the structure, antioxidant properties, hypoglycemic effects, and immunomodulatory activities of FCPS were evaluated. FCPS was characterized as irregular, rough-surfaced, flaky materials consisting of pyran-type polysaccharides with α- and β-glycosidic linkages, and composed of multiple monosaccharides and only one homogeneous concentrated polysaccharide component (FCPS1) with a molecular weight of 4.224 × 104 Da. The results suggested FCPS exhibited remarkable antioxidant activity in vitro, as evidenced by improved cell viability and reduced the reactive oxygen species (ROS) levels. Meanwhile, FCPS effectively improved liver-related insulin resistance by promoting glucose consumption in hepatocytes and activated the immune response through activation of murine bone marrow-derived dendritic cells (DCs) and upregulation of interleukin 6 (IL6) and interleukin 12 (IL-12) expression. The findings demonstrate the efficacy of the UAE technique in isolating FCPS with biological functionality and FCPS could potentially serve as a beneficial organic antioxidant source and functional food, carrying important implications for future studies.
1. Introduction
Ficus carica L., a member of the genus Ficus in the family Moraceae, is a diminutive deciduous tree encompassing a vast array of over 800 different species, and predominantly thriving in tropical and temperate regions [1]. F. carica has been cultivated in China for a long time, includeing “Xinjiang Zaohuang”, an indigenous early variety found in Xinjiang, China [2]. This variety exhibits a summer fruit ripening period in early July and an autumn fruit ripening period in mid to late August. Additionally, the weight of a single fruit can reach 50–70 g, with a high soluble solids content ranging from 15 % to 17 %. As an influential fruit crop, F. carica possesses a diverse range of nutrients such as vitamins (7.1 % thiamine, 6.2 % riboflavin per 100 g), minerals (30 % iron, 15.8 % calcium, 14 % potassium per 100 g), amino acids (with aspartic acid and glutamine being the most abundant), protein, dietary fiber, and other essential components [3], [4], [5]. Additionally, it also contains polysaccharides, polyphenols, flavonoids, organic acids, psoralen, and various other bioactive compounds [3], [5], [6], [7], [8]. Notably, F.carica is devoid of sodium, fat, and cholesterol [4].
Plant polysaccharides, which consist of multiple monosaccharides linked by glycosidic bonds, are integral constituents of plant cell walls and serve as energy stores. These macromolecules play crucial roles in diverse biological processes, including biosynthetic reactions, intercellular recognition and communication, signaling, cell proliferation, immunity, and defense [9], [10], which exhibit a range of biological functions, such as antioxidative properties [9], [11], anticancer activity [12], anti-inflammatory effects [12], [13], antidiabetic effects [14], immunomodulation [12], and absence of cytotoxicity. Despite these encouraging properties, the lack of research on the structural attributes and biological functionalities of F.carica polysaccharide (FCPS) hinders its advancement and utilization. Hence, it is crucial to explore the FCPS extraction methods and identify the most effective bioactive polysaccharides to enhance the advancement of functional food products.
The conventional method of extracting plant polysaccharides, which involves hot water extraction, has specific drawbacks including extended extraction durations, increased temperatures, and excessive use of solvents. These factors negatively impact the biological effectiveness of the polysaccharides [9], [15]. Ultrasonic-assisted extraction (UAE) has become a potent method for extracting botanicals among these techniques [16]. This approach affects the chemical or steric structure of polysaccharides through mechanically emulsifying and cavitating plant cells, resulting in the extraction of bioactive polysaccharides [17], [18]. The advantages of UAE include reduced processing times, lower temperatures, minimized reagent consumption, and increased potency. In addition, UAE has the potential to reduce the molecular weight (MW) and viscosity of polysaccharides with high MW, thereby exposing the antioxidant active groups, resulting in superior DPP scavenging and Fe3+ reducing power compared to hot water extraction [9], [19]. Multiple research studies have indicated that this technology is appropriate for large-scale implementation in industries [20], [21].
Response surface methodology (RSM) is a statistical technique that employs a series of quadratic regression equations to examine experimental data and assess the impacts and interrelationships of various influential factors, aiming to identify the most favorable process parameters [22]. Its widespread application in various fields, such as agriculture, biology, food science, and chemistry, can be attributed to its high accuracy and efficiency in cost reduction and process optimization.
The ultrasound-assisted hot water extraction method was utilized to acquire FCPS in this research. The structure and in vitro antioxidant capacity of FCPS were characterized. Given the notable effectiveness and efficiency of UAE, we utilized hydrogen peroxide (H2O2) and palmic acid (PA) -induced HepG2 cell models and DCs to explore the antioxidant properties, hypoglycemic effects, and immunomodulatory activities of FCPS.
2. Materials and methods
2.1. Materials
The fruits of Ficus carica were obtained from Xinjiang, China. Human liver cancer cell HepG2 was purchased from the stem cell bank of Chinese Academy of Sciences (Shanghai, China). 1-diphenyl-2-picrylhydrazine free radical (DPPH), and Hydroxyl radical test kit was purchased from biyuntian Biotechnology company (Shanghai, China). Granulocyte-macrophage colony-stimulating factor (GM-CSF), was purchased from Peprotech (USA). Ascorbic acid (vitamin C, Vc) was purchased from Shanghai Blue season Technology Development company (Shanghai, China). Dimethyl sulfoxide (DMSO), cisplatin (DDP), 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) and 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) were purchased from sigma (St. Louis, USA). Phosphate buffer (PBS) and pancreatin were purchased from GIBCO (California, USA). Tumor necrosis factor receptor superfamily member 5 (CD40), Allophycocyanin (APC) linked B-lymphocyte antigen B7-2(APC-CD86), IL-6 and IL-12 were purchased from Elabscience (Wuhan, China).
2.2. The extraction methods of FCPS
2.2.1. The ultrasonic-assisted extraction of FCPS
Briefly, 5.0 g of the dried fruit powder of F.carica was treated with a petroleum ether solution at a 5:1 vol ratio in a water bath set at 60 ℃ for 2 h, and then suspended in an anhydrous ethanol solution at the same 5:1 vol ratio to eliminate lipids and others impurities, respectively. Proteins were removed using the sevag method [23]. According to the method reported in literature [24], the sediment was mixed with distilled water at a designated ratio of liquid to solid (1:10–1:35 g/mL) and subjected to ultrasonic treatment using a 300 W, 40 kHz ultrasonic cleaning bath (XM-P30H, 30L, the internal dimensions: 500 × 300 × 200 cm, Kunshan Ultrasonic Instrument Co., Ltd., Jiangshu Province, China) for 25 min at a predetermined temperature (range from 50 to 100℃) and a variable number of ultrasonic extractions (1–6 times). Following ultrasonic treatments, the mixture was extracted within a specified duration (60–210 min) and subsequently concentrated into a paste using rotary evaporation (Rotavapor® R-100, BUCHI, Switzerland). Afterwards, the resulting concentrate was mixed with a solution containing four times volume of 80 % ethanol and left to settle at 4℃ for 24 h. The solution was then centrifuged at 4000 rpm for 10 min. Then, the FCPS were collected, freeze-dried and stored at −20 ℃.
2.2.2. The conventional hot water extraction of FCPS
The conventional extraction (without ultrasound, CE) method, as described in section 2.2.1, was utilized to extract FCPS. However, instead of utilizing ultrasound-assisted extraction, the extraction process was subjected to directly heating in a water bath at 70 °C for 2 h after pre-treatment, maintaining a solid–liquid ratio of 1:20 (g/mL). A total of four extractions were performed.
2.2.3. The microwave-assisted extraction (MAE) of FCPS
Similar to the methodology employed in UAE experiments, the pre-treated F.carica fruits powder (5.0 g) was subjected to extraction using distilled water at a solid-to-liquid ratio of 1:20 (g/mL). The extraction process was carried out using a microwave extraction apparatus (M1-L213B, 20L, the internal dimensions: 307 × 307 × 312 cm, Midea Microwave, Guandong Province, China) for 25 min at 500 W. Subsequently, the mixture was further extracted at 70 °C for 2 h, following the same procedure as the conventional extraction system.
2.2.4. The enzyme-assisted extraction (EAE) of FCPS
The pre-treated samples (5.0 g) were immersed in 100 mL of distilled water and subsequently subjected to extraction with 1 % cellulase enzyme at 55 °C for 1 h. The resulting mixture was rendered inactive at 80 °C for 10 min, followed by heating in a water bath at 70 °C for 2 h, employing the identical procedure as the conventional extraction system.
2.3. The single-factor experimental design
The FCPS yield was determined through a single-factor experimental design, that identified the critical factors and their corresponding levels. Detailed information on the four factors and experimental levels, including the ratio of solid–liquid, the durations of extraction, ultrasonic temperature, and the number of ultrasonic extractions, were provided in Table 1, which highlighted their impact on the yield of FCPS.
Table 1.
Single factorial design factors and levels.
| Factors | Levels | |||||
|---|---|---|---|---|---|---|
| The ratio of solid-to-liquid (g/mL) | 1:10 | 1:15 | 1:20 | 1:25 | 1:30 | 1:35 |
| The durations of extraction (min) | 60 | 90 | 120 | 150 | 180 | 210 |
| Ultrasonic temperature (℃) | 50 | 60 | 70 | 80 | 90 | 100 |
| The number of ultrasonic extractions (times) | 1 | 2 | 3 | 4 | 5 | 6 |
FCPS yield (%) = [total FCPS weight (g)/ dried powder weight (g)] × 100 % [25].
2.4. Response surface optimization design
The UAE conditions were optimized using a three-factor, three-level Box-Behnken design (BBD) in Design Expert® 8.0.6, utilizing the findings from the single-factor experiments, with the response variable being FCPS yield. Table 2 displayed the experimental factors and levels, namely the ratio of solid–liquid, ultrasonic temperature, and the number of ultrasound extractions.
Table 2.
RSM factors and levels.
| Factors | Unit |
Levels |
||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: The ratio of solid-to-liquid | g/mL | 15 | 20 | 25 |
| B: Ultrasonic temperature | ℃ | 60 | 70 | 80 |
| C: The number of ultrasonic extractions | times | 3 | 4 | 5 |
2.5. Scanning electron microscopy (SEM) analysis
The SEM analysis (SU8010, Hitachi, Tokyo, Japan) was conducted to examine the microstructure of FCPS, following the previously described method [26]. A thin gold layer was applied to a small amount of dried FCPS, and images were taken at different magnifications ranging from 500× to 30,000×.
2.6. Fourier transform infrared (FT-IR) spectral analysis
FT-IR spectroscopy was used to characterize the structure of FCPS, as per the method mentioned earlier [27]. In particular, a mixture comprising 4.0 mg of FCPS and 300 mg of potassium bromide (KBr) was made, and the resultant tablets underwent FT-IR spectroscopy within the 400 to 4000 cm−1 range. The scans were conducted 64 times with a resolution of 4 cm−1, and the data were analyzed using OPUS 6.5 software.
2.7. The monosaccharide composition determination of FCPS
Polysaccharides were subjected to analysis of their monosaccharide composition using high performance liquid chromatography (HPLC) [28]. 2.0 mg of FCPS was accurately weighed and added into 1.0 mL of 4.0 mol/L trifluoroacetic acid (TFA) in a hydrolysis tube, where it underwent hydrolysis at 110℃ for 5 h. Next, 0.1 mL of the resulting hydrolysate was dried in a vacuum oven at 60 ℃ for 2 h. Subsequently, 0.05 mL of 0.3 mol/L NaOH and 0.05 mL of PMP (1-phenyl-3-methyl-5-pyrazolone) methanol solution were added. The tube was then filled with nitrogen and placed in a water bath at 70 ℃ for 60 min. After cooling, 0.05 mL of 0.3 mol/L HCl, 0.75 mL of ultrapure water and 1.5 mL of chloroform were added to the solution, and the aqueous phase was collected and filtered after shaking and standing for stratification. 10 μL of the samples were analysed on an Agilent 1260 HPLC system with a C18 column (5 μm, 4.6 mm × 250 mm) and an Agilent 1260-DAD detector. Retention times and peak areas were utilized to determine monosaccharide content and composition of FCPS compared to monosaccharide standards.
2.8. The MW determination of FCPS
High performance gel permeation chromatography (HPGPC) was used to determine the uniformity and MW of polysaccharides [27]. To estimate the relative MW of FCPS, glucan standards with different MW (10–2000 kDa) were utilized to construct standard curves.
2.9. Free radical scavenging assay
100 μL DPPH-ethanol solution (0.1 mmol/L) was added to 100 μL of various concentrations of FCPS solutions. After incubating in darkness for 10 min, the measurement was taken at 517 nm using a microplate reader. The scavenging capacity of FCPS on hydroxyl free radicals was measured following the instructions. Positive, blank, and background controls were established using Vc (0.25 mg/mL), distilled water, and anhydrous ethanol, respectively.
2.10. Effect of FCPS against oxidative damage in H2O2-induced HepG2 cells
Establishment of an oxidative damage model HepG2 cells were incubated in MEM medium supplemented with 10 % FBS. Cells were exposed to various levels of H2O2 (100, 200, 400, 800, 1200, and 1600 μmol/L) for a duration of 6 h and 12 h. Following the PBS rinse, 100 μL of MTT solution was added and incubated at 37℃ for 4 h. Next, the formazan was dissolved by adding 150 μL DMSO and subsequently measuring the absorbance at 490 nm. Cell viability (%) was calculated. Furthermore, the MTT assay [29] was employed to assess the cytotoxicity of FCPS (200, 400, 800, 1600, and 3200 μg/mL) and Vc (100, 200, 400, 800 μg/mL) towards HepG2 cells.
The protective effect of FCPS against H2O2-induced HepG2 cells Briefly, cells exposed to H2O2 was evaluated by subjecting the cells to 24 and 48 h treatment with FCPS, followed by co-incubation with an H2O2 solution for 12 h, and subsequently assessing the cell viability.
Cell viability (%) = (OD sample 490 – OD blank 490)/ (OD untreated 490 – OD blank 490) × 100, (1)
2.11. Determination of the total ROS generation
The methodology described above was followed to perform cell culture, FCPS, and H2O2 treatments. Negative controls consisted of untreated cells, while positive controls were represented by Vc. After collecting and double washing with PBS, DCFH-DA fluorescent probe was utilized in accordance with the instructions provided in the kit. The levels of ROS were detected by Flow cytometry.
2.12. Effect of FCPS on glucose uptake in IR-HepG2 cells
Establishment of insulin resistance (IR) model HepG2 cells were exposed to PA (0.1 mM, 0.2 mM, and 0.4 mM) for 24 h. A solvent control using 1 % bovine serum albumin (BSA) was utilized. Cell proliferation viability was evaluated through the MTT assay, while glucose consumption was measured according to a glucose assay kit.
The ameliorating effect of FCPS against insulin resistance in HepG2 cells Cells were exposed to 0.1 mmol/L PA along with various concentrations of FCPS (200–3200 μg/mL) for 24 and 36 h. Metformin (Met) was used as a positive control. Glucose consumption was measured as previously described [30].
2.13. Animals and ethics statement
C57BL/6 mice, aged six weeks, were acquired from the laboratory animal center of Xinjiang Medical University (Urumqi, China). The mice were maintained in the Xinjiang University Animal Facility with controlled temperature and light. The Animal Ethics Committee of Xinjiang Key Laboratory of Biological Resources and Genetic Engineering (BRGE-AE001) approved all animal experiments [30].
2.14. Effect of FCPS on the maturation of dendritic cells (DCs) maturation
GM-CSF was used to generate immature DCs from C57BL/6 mouse bone marrow cells [31]. Lipopolysaccharides (LPS, 40 ng/mL) was used as a positive control for DCs on the 7th day following FCPS treatment. Harvested cells were labeled with monoclonal antibodies that target mouse CD40 and APC-CD86. Afterward, the CytoFLEX flow cytometer from Beckman Coulter was used to examine all samples, and the data obtained were then analyzed with FlowJo software (v7.6). After collecting the supernatant, the levels of cytokines (IL-6 and IL-12p40) were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, China). OD450 was measured using a microplate reader.
2.15. Statistical analysis
All data were presented using the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was utilized for conducting the statistical analyses. P < 0.05 was used to define statistical significance.
3. Results
3.1. The single-factor experiment of FCPS extraction
The ratio of the solid-to-liquid is of utmost importance in the process of ultrasonic extraction of plant polysaccharides. It needs to be controlled within certain limits. According to Fig. 1a, when the ratio was increased, the FCPS yield also increased, peaking at 6.073 % with a ratio of 1:20 (g/mL). However, a ratio exceeding 1:20 (g/mL) led to a gradual decrease in FCPS yield. The optimal solid-to-liquid ratio facilitated a higher concentration gradient of the solvent across the F. carica cell membrane, increasing the polysaccharide diffusion coefficient and facilitating rapid dissolution [32]. However, as the ratio continued to increase, the excess solvent weakened the ultrasound energy, resulting in decreased FCPS yield.
Fig. 1.
The impact of ultrasonic extraction variables on FCPS yield. (a) The ratio of solid-to-liquid, (b) The duration of extraction, (c) Ultrasonic temperature, (d) Number of ultrasonic extractions.
Polysaccharide leaching is time-dependent, as insufficient extraction times may result in low polysaccharide dissolution rates. Conversely, prolonged extraction durations can result in the degradation of polysaccharide sugar chain, dissolution of impurities, and a reduction in extraction efficiency. As depicted in Fig. 1b, FCPS yield significantly rose as the extraction duration increased, peaking at 6.280 % after 120 min. However, beyond this time, the FCPS yield gradually declined, aligning with earlier research [33].
At lower temperatures (below 60–70 °C), the desorption properties and solubility of the substance in the extraction solvent are augmented with increasing temperature, resulting in heightened diffusion rate of the solvent through the tissue matrix and increased yield of UAE. Nevertheless, as the temperature continues to rise, the reduction in solvent surface tension caused by the weakening of cavitation effect at high temperatures significantly hampers mass transfer of the components to be extracted, leading to a decrease in polysaccharide yield [34], [35], [36]. The result indicated that FCPS yield exhibited a notable increase as the ultrasonic temperature rose from 50 ℃ to 70 ℃, reaching the maximum yield of 6.710 % at 70 ℃. However, FCPS yield decreased when the temperature exceeded 70 ℃ (Fig. 1c). This is consistent with previous reports [37], [38].
Enhancing the number of ultrasonic extractions within suitable frequency and duration parameters not only improves the efficiency and quality of polysaccharide extraction, but also guarantees the extraction of polysaccharides in its entirety. The FCPS yield progressively rose, as the number of ultrasonic extractions increased, reaching a maximum of 7.902 % following four extractions, as shown in Fig. 1d. Nevertheless, once the number of extractions surpassed four, FCPS productivity declined significantly.
3.2. 7 Response surface optimization and model validation
3.2.1. 8 The design and results of experiments using RSM
A total of 17 experiments were conducted to optimize the process conditions of FCPS. These experiments followed the three-factor and three-level design, incorporating the principles of BBD. The study design consisted of 12 analytical experiments (experiments 1–12) and 5 central experiments (experiments 13–17), considering the findings from the single-factor experiments. The data were examined using Design Expert v8.0.6 software, and the results are displayed in Table 3.
Table 3.
The experiment design and results of RSM.
| Runs | A | B | C | Actual value of Y | Predicted value of Y |
|---|---|---|---|---|---|
| 1 | 15 | 60 | 4 | 7.49 | 7.51 |
| 2 | 25 | 60 | 4 | 7.40 | 7.38 |
| 3 | 15 | 80 | 4 | 7.19 | 7.20 |
| 4 | 25 | 80 | 4 | 7.45 | 7.43 |
| 5 | 15 | 70 | 3 | 7.21 | 7.20 |
| 6 | 25 | 70 | 3 | 7.28 | 7.31 |
| 7 | 15 | 70 | 5 | 7.64 | 7.61 |
| 8 | 25 | 70 | 5 | 7.60 | 7.61 |
| 9 | 20 | 60 | 3 | 7.43 | 7.42 |
| 10 | 20 | 80 | 3 | 7.22 | 7.21 |
| 11 | 20 | 60 | 5 | 7.69 | 7.70 |
| 12 | 20 | 80 | 5 | 7.64 | 7.65 |
| 13 | 20 | 70 | 4 | 7.97 | 7.96 |
| 14 | 20 | 70 | 4 | 7.96 | 7.96 |
| 15 | 20 | 70 | 4 | 7.98 | 7.96 |
| 16 | 20 | 70 | 4 | 7.92 | 7.96 |
| 17 | 20 | 70 | 4 | 7.96 | 7.96 |
A. the ratio of solid-to-liquid (g/mL). B. ultrasonic temperature (℃). C. the number of ultrasonic extraction (times). Y. FCPS yield (%).
3.2.2. Establishment of regression models and variance analysis of FCPS yield
Table 4 presented the ANOVA and significance test results for the experimental models. The model was statistically significant with an F-value of 206.32 and a P-value of less than 0.01. The model demonstrated a strong correspondence with the actual test, as indicated by the mismatch term having an F value of 1.95 and a P value exceeding 0.05, along with a coefficient of determination R2 of 0.9962. The corrected coefficient of determination R2Adj was 0.9914, which is close to R2, suggesting the high predictive accuracy and versatility of the model. The regression equation was formulated as follows:
Table 4.
The ANOVA of response surface quadratic model for FCPS yield.
| Source | Sum of squares | df | Mean square | F-value | P value | Significance |
|---|---|---|---|---|---|---|
| Model | 1.31 | 9 | 0.15 | 206.32 | <0.0001 | ** |
| A | 5.30E-03 | 1 | 5.30E-03 | 7.51 | 0.0289 | * |
| B | 0.033 | 1 | 0.033 | 46.21 | 0.0003 | ** |
| C | 0.25 | 1 | 0.25 | 360.34 | <0.0001 | ** |
| AB | 0.032 | 1 | 0.032 | 45.36 | 0.0003 | ** |
| AC | 2.60E-03 | 1 | 2.60E-03 | 3.68 | 0.0965 | |
| BC | 6.97E-03 | 1 | 6.97E-03 | 9.87 | 0.0163 | * |
| A2 | 0.43 | 1 | 0.43 | 604.18 | <0.0001 | ** |
| B2 | 0.28 | 1 | 0.28 | 394.07 | <0.0001 | ** |
| C2 | 0.17 | 1 | 0.17 | 245.93 | <0.0001 | ** |
| Residual | 4.95E-03 | 7 | 7.06E-04 | |||
| Lack of fit | 2.94E-03 | 3 | 9.79E-04 | 1.95 | 0.2635 | |
| Pure error | 2.01E-03 | 4 | 5.02E-04 | |||
| Cor total | 1.32 | 16 | ||||
| R2 = 0.9962 R2Adj = 0.9914 R2Pred = 0.9619 C.V.%=0.35 | ||||||
*: p < 0.05; **: p < 0.01.
Y1 = 7.96 + 0.026A-0.064B + 0.18C + 0.09AB-0.025AC + 0.042BC-0.32A2-0.26B2-0.2C2, (2)
The regression analysis unveiled a noteworthy non-linear correlation between variables A, B, and C and the yield of FCPS. Consequently, the P-values for the linear variables, along with the interaction variables AB and BC, and the quadratic variables A2, B2, and C2 were all below 0.05, signifying the significant impact of these factors on the FCPS yield. The impact of ultrasound on FCPS extraction was effectively demonstrated, with the highest significant influence observed in the number of ultrasonic extractions (C) followed by ultrasonic temperature (B) and the ratio of solid-to-liquid (A)
3.2.3. Response surface analysis of FCPS yield
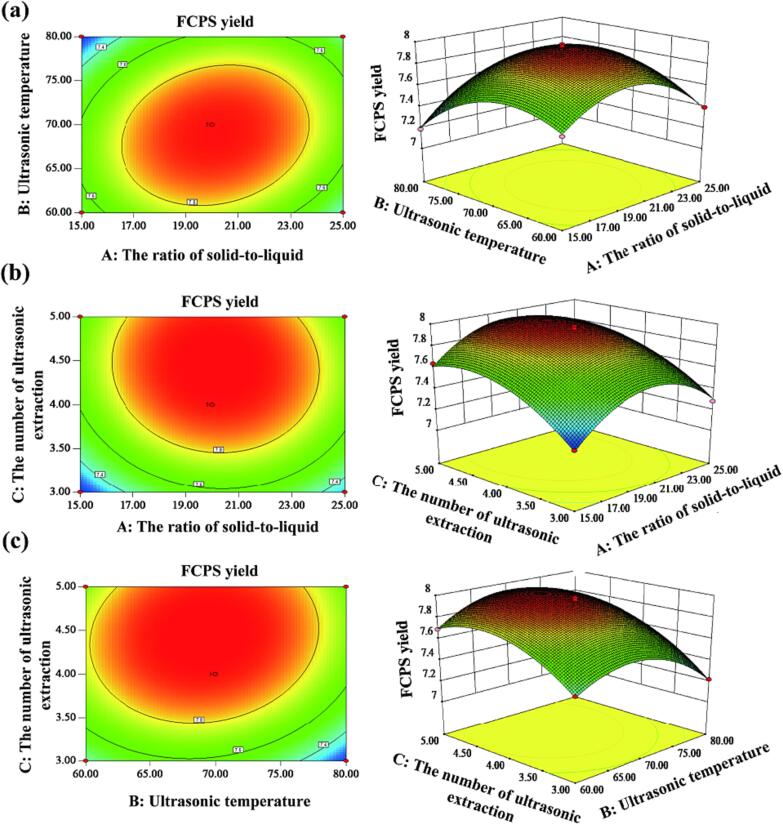
The relationship between each factor and its interaction effect on the FCPS yield was visually illustrated in Fig. 2. The strength and significance of interactions can be displayed through contour plots and 3-D plots. The interaction between the variables becomes stronger as the response surface becomes more elliptic. Experimental settings show a consistent decline in the response area in 3-D graphs, with a pinnacle indicating an ideal solution [39], [40].
Fig. 2.
Analysis of the yield of FCPS using response surface (a) The interaction of variables A and B. (b) The interaction of variables A and C. (c) The interaction of variables B and C.
The findings indicated the notable impact of the solid-to-liquid ratio (A) and ultrasonic temperature (B) by revealing the existence of an elliptical contour line and a steep response surface (Fig. 2a),whereas the contour line shape of A and C was close to a circle and the response surface was smooth, suggesting that the interaction was considered to be insignificant. (Fig. 2b). In contrast, the interaction between B and C on FCPS yield was significant (Fig. 2c). The findings aligned with the analysis of variance.
3.2.4. Optimal extraction process determination and validation
The quadratic model was used to determine the ideal conditions for prediction. These conditions included the ratio of solid-to-liquid (A) of 1:20.06 (g/mL), an ultrasonic temperature (B) of 69.13 ℃, and the number of ultrasonic extraction (C) of 4.43 times. The theoretical optimal FCPS yield was calculated as 7.99 ± 0.02 %. The actual test conditions were then modified to A = 1:20 (g/mL), B = 70 ℃, and C = 4 times as the optimized conditions. The average FCPS yield was 7.97 ± 0.05 %. These results demonstrated the accuracy and reliability of the model, highlighting its potential application in optimizing FCPS extraction (Table 5).
Table 5.
Optimization of extraction conditions of FCPS.
| Extraction conditions |
A. the ratio of solid-to-liquid (g/mL) |
B. ultrasonic temperature (℃) |
C. the number of ultrasound extractions (times) |
|
|---|---|---|---|---|
| Prediction value | 20.06 | 69.13 | 4.43 | 7.99 ± 0.02 % |
| Actual value | 20 | 70 | 4 | 7.97 ± 0.05 % |
3.2.5. SEM analysis of FCPS
Polysaccharides are complex biomolecules that contain more structural information compared to proteins, nucleic acids, and lipids [41]. The physical properties of polysaccharides, such as solubility, release rate, and viscosity, are closely related to their structural morphology [42]. The microstructures of FCPS were observed by SEM at different magnifications of 500×, 1,000×, 10,000×, and 30,000× (Fig. 3). The FCPS exhibited unevenly distributed slices with a rough surface, and multiple surface perforations were observed after the UAE. Furthermore, the thickness of the FCPS was measured to be approximately 15 μm at a magnification of 10,000×.
Fig. 3.
SEM analysis of FCPS with different magnifications, including 500× (a), 1 000× (b), 10 000× (c), and 30 000× (d).
3.2.6. FT-IR analysis of FCPS
Comprehending the relationship of the structure–activity of polysaccharides is essential as their intricate compositions of glycosyl and glycosidic linkages dictate their diverse biological functions [43]. In Fig. 4, the FCPS infrared spectrum exhibited a prominent and broad absorption peak at 3401.535 cm−1, which corresponds to the O-H stretching vibration [44]. Furthermore, the identification of a feeble peak absorbed at 2929.922 cm−1 suggested the existence of the stretching vibration of C-H linkage in polysaccharides [45]. The presence of carboxyl group in FCPS was primarily suggested by the weak absorption peak observed at 1745.600 cm−1 [46]. Additionally, the C O groups exhibited asymmetric stretching vibration bands at 1614.094 cm−1 [47]. Moreover, the peaks observed at 1416.154 cm−1 were a symmetric stretching mode of deprotonated —COO— groups [48], and the peak at 1259.717 cm−1 was attributed to the S O stretching vibration of the sulfate group [49], [50], [51], [52], [53], [54]. These results indicated that FCPS included a carbonyl moiety and was made up of sulfated polysaccharides.
Fig. 4.
Analysis of FCPS using FT-IR spectra.
The three peaks observed within the 1000–1200 cm−1 range were additionally caused by the elongation oscillation of the C-O-C glycosidic bonds, as well as the C-O-H on the pyranose ring [54]. Furthermore, the β-pyranose arrangement was determined for the significant at 915.357 cm−1 [55]. The α-glycosidic bond and C-H variable angle vibration in the furan ring were associated with a faint absorption peak at 818.778 cm−1, whereas the absorption at 633.041 cm−1 was attributed to the C-H bending vibration in the sugar chain and aromatic group [56]. FT-IR analysis revealed that FCPS displayed distinct functional groups of polysaccharides, specifically pyranose, containing both α-type and β-type glycosidic linkages. This observation is highly relevant to its potent biological activity.
Furthermore, the structure of the FCPS obtained from CE, MAE, and EAE was characterized and compared to UAE (Fig. S2). The FTIR results revealed that these characteristic absorption peaks at approximately 3400 cm−1, 2930 cm−1, and 1012 cm−1 were typical for polysaccharides. However, it was observed that the intensity of the absorption peaks at 1259 cm−1 was notably diminished in FCPS extracted by CE, MAE, and EAE when compared to UAE. Concurrently, in the infrared spectrum of MAE and EAE, the absence of absorption peaks at 915 cm−1 distinguished it from UAE. These results indicated various extraction techniques could alter the composition of functional sulfate group and β-type glycosidic bond structure of FCPS, potentially leading to a discernible variations in its biological activity.
3.3. The molecular weight analysis of FCPS
The Mw of polysaccharides exerts a substantial influence on their bioactivity [57]. The determination of FCPS’s Mw and dispersion index was conducted using HPGPC. As depicted in Table 6 and Fig. 5, the blue line represented the differential signal, reflecting the concentration, while the red line represented the multi-angle laser light scattering signal, which mainly reflects the Mw. The results revealed the presence of a peak at the retention time (tR) of 9.2 min–33.5 min, indicating that FCPS consisted of a single polysaccharide component (FCPS1). Moreover, FCPS1 displayed a relatively limited range of molecular weights, having an average Mw of 4.224 × 104 Da, a number average molecular weight (Mn) of 3.008 × 104 Da, a peak molecular weight (Mp) of 2.680 × 104 Da, and a dispersion coefficient D (Mw/Mn) of 1.396.
Table 6.
The Mw distribution of FCPS.
| Molecular weight | Distribution |
|---|---|
| The average molecular weight (Mw) | 4.224 × 104 Da |
| The number average molecular weight (Mn) | 3.008 × 104 Da |
| The peak molecular weight (Mp) | 2.680 × 104 Da |
| The dispersion coefficient D (Mw/Mn) | 1.396 |
Fig. 5.
The Mw analysis of FCPS.
3.4. The monosaccharide composition of FCPS
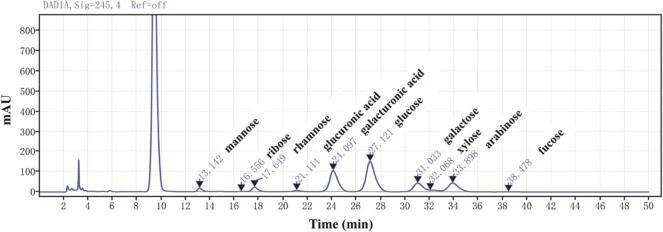
Research have found that the antioxidant potential of polysaccharides can be influenced by the particular composition and molar ratio of monosaccharides [58]. The main components of FCPS were glucose and galacturonic acid, along with arabinose, galactose, and trace amounts of rhamnose, mannose, xylose, glucuronic acid, fucose, and ribose (Fig. 6). The contents of these monosaccharides were 119.19: 98.53: 47.96: 36.04: 19.34: 7.49: 5.84: 4.59: 2.05: 1.70, respectively. As can be seen from the peak area, the monosaccharide molar ratio is 43.80: 25.94: 18.00: 13.42: 5.04: 2.90: 2.18: 1.68: 0.24:0.49. (Table S1). The results suggested that the predominant constituents comprising the FCPS sugar chain backbone are glucose and galacturonic acid.
Fig. 6.
The monosaccharide composition analysis of FCPS.
3.5. In vitro free radical scavenging capacity of FCPS
Excessive generation of free radicals can cause cell and tissue damage, leading to diseases such as cancer, aging, diabetes, and immune dysfunction [59]. DPPH, a stable free radical centered on nitrogen, is commonly utilized for the evaluation of antioxidant capability of active compounds [60]. The scavenging rate of the DPPH radical gradually increased with increasing FCPS concentration, reaching 72.74 % at 2.0 mg/mL concentration (Fig. 7a), indicating that FCPS extracted by UAE had robust DPPH radicals scavenging ability, aligning with findings from prior research [24]. This phenomenon could be attributed to the presence of an unpaired electron at the center of the nitrogen atom in the DPPH molecule, which confers stability and the ability to accept an electron or hydrogen ion. Upon interaction with FCPS, the DPPH radicals were effectively reduced as FCPS captured the radicals and supplied them with an electron or hydrogen atom, leading to the formation of stable and inert compounds. As a result, the length of the radical chain was diminished and the oxidation reaction initiated by these radicals was hindered. Consequently, a direct scavenging effect of FCPS on the radicals was achieved, ultimately leading to in a decrease in oxidative harm imposed on cellular structures [11], [61], [62], [63].
Fig. 7.
The impact of FCPS on the activity of free radicals. (a) The capacity of scavenging DPPH radicals. (b) The capacity of scavenging hydroxyl radicals. *** p < 0.001, comparison between FCPS and untreated.
Hydroxyl free radicals, considered the most reactive oxygen species in the body, interact with proteins, DNA, and biofilms, resulting in cellular damage and lipid oxidation [64]. According to Fig. 7b, when the FCPS concentration was raised to 1.0 mg/mL, the scavenging rate of hydroxyl radical reached a peak of 37.96 %. This suggested that FCPS facilitated the transfer of hydrogen electrons to interact with the hydroxyl group, and could form complexes with metal ions, such as ferrous iron ions, which were essential for the generation of hydroxyl radicals. The process hindered lipid peroxidation and suppressed the production of reactive oxygen species, thus resulting in the efficient elimination of hydroxyl radicals [62], [63], [65]. These findings demonstrated that FCPS efficiently extracted by UAE exhibits strong antioxidant activity, thus suggesting its potential as a viable natural antioxidant.
To further validate the impact of UAE on the extraction of FCPS, the yield of FCPS extracted from CE, EAE, MAE and their influence on DPPH radical activity were assessed. The result revealed that the yield of FCPS achieved through the aforementioned three methods were 4.70 %, 8.64 %, and 7.50 %, respectively (Table S1). Additionally, the semi-inhibitory concentration of DPPH radical scavenging (IC50) of FCPS with UAE was 0.18 mg/mL, whereas the IC50 values for the other three methods were 2.0 mg/mL, respectively (Fig. S1). The EAE method resulted in a higher yield, however, the antioxidant activity was comparatively weaker than that of UAE method. These results confirmed that UAE exhibits the most pronounced impact on the yield and efficiency of FCPS.
3.6. FCPS reversed the inhibition of cell growth in H2O2-induced HepG2 cells
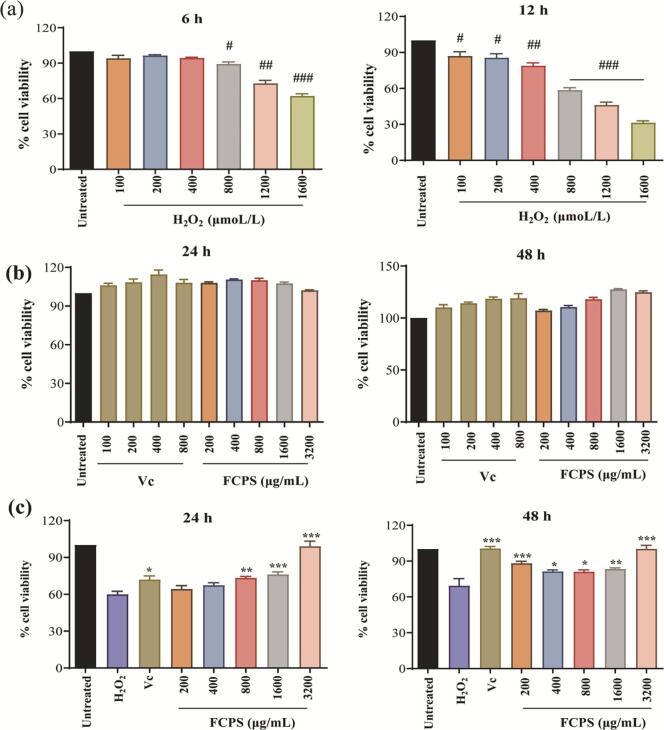
To assess the antioxidant effectiveness of FCPS, a model of oxidative damage was established in HepG2 cells utilizing H2O2. The concentration of H2O2 was raised to 800 μM, leading to an inhibitory effect that was half-maxima (Fig. 8a). Hence, a treatment duration of 12 h with 800 μM H2O2 was chosen for model establishment.
Fig. 8.
The impact of FCPS on the growth of H2O2-induced HepG2 cells. (a) A damage model establishment by H2O2 for 6 and 12 h. (b) Cytotoxic effect of Vc and FCPS for 24 and 48 h. (c) FCPS treatment for 24 and 48 h, respectively. The significance levels were #p < 0.05, ##p < 0.01, and ###p < 0.001, when comparing H2O2 to the untreated group. *p < 0.05, **p < 0.01, and ***p < 0.001, when comparing FCPS to the H2O2 group.
Cell viability, which represents the cytotoxicity of samples, is a critical parameter in toxicology experiments [66]. As shown in Fig. 8b, both FCPS and Vc did not have a significant impact on the growth of HepG2 cells at 24 or 48 h, when compared to the untreated group. Consequently, further examination was conducted on FCPS concentrations <3200 μg/mL and Vc concentrations <800 μg/mL.
In Fig. 8c, the viability of H2O2-induced HepG2 cells was not significantly improved by 200 μg/mL and 400 μg/mL of FCPS treatment. Nonetheless, 800 μg/mL, 1600 μg/mL, and 3200 μg/mL of FCPS exhibited a significant enhancement in cellular proliferation within 24 h. Notably, the cell survival rate experienced a substantial boost of 122.26 %, 127.06 %, and 165.22 %, respectively, surpassing that of the Vc group (119.92 %). Moreover, FCPS demonstrated a notable enhancement in cell growth in H2O2-induced HepG2 cells after 48 h.
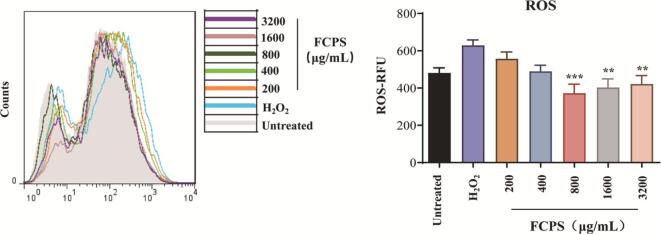
3.7. FCPS suppressed the levels of intracellular ROS in H2O2-induced HepG2 cells
ROS, also known as reactive oxygen species, are highly active free radicals that are produced within the organism due to both internal and external stimulation during cellular metabolism. Excessive accumulation of ROS leads to peroxidation of biomacromolecules, including proteins, nucleic acids, and lipids, thereby inducing oxidative damage and affecting cellular viability [67]. Fig. 9 demonstrates that FCPS effectively reduced the levels of ROS in H2O2-induced HepG2 cells. The levels of ROS caused by H2O2 were considerably higher in comparison with the untreated group. Nevertheless, FCPS treatment at concentrations of 800 μg/mL, 1600 μg/mL, and 3200 μg/mL resulted in a noteworthy decrease in ROS levels.
Fig. 9.
The impact of FCPS on the levels of intracellular ROS in H2O2-induced HepG2 cells.
It was noteworthy to mention that the reaction kinetics between FCPS and ROS levels displayed nonlinearity, which was a cause for greater concern. Specifically, at lower concentrations, FCPS exhibited a rapid ability to interact with ROS, thereby facilitating effective scavenging. However, as the concentration of FCPS increased, a saturation effect or competitive reaction might occur, resulting in a potential cessation in the decrease of ROS activity or even the maintenance of a stable level. This is consistent with previously reported studies [68]. The findings indicated that FCPS, acting as a natural antioxidant, had the potential to enhance the survival of HepG2 cells and alleviate the cellular harm caused by ROS.
3.8. FCPS enhanced glucose metabolism in IR-HepG2 cells
Excessive production of ROS damages insulin secretion and triggers the development of diabetes mellitus (DM) and IR. Insulin resistance (IR), a typical mechanism of DM, is marked by diminished responsiveness of specific tissues, such as the liver, to insulin, resulting in compromised glucose tolerance [69]. PA leads to fatty acid accumulation and fatty liver development, which disrupts insulin signaling and impairs glucose metabolism, leading to hepatic insulin resistance. The results showed that impaired glucose uptake in HepG2 cells was significantly induced using 0.1 mM and 0.2 mM of PA, without any inhibitory impact on cell proliferation within 24 h (Fig. 10a). Different concentrations of FCPS were found to greatly enhance glucose consumption by HepG2 cells in a dose-dependent manner, as observed in Fig. 10b. These results suggested that FCPS effectively ameliorated PA-induced liver-related insulin resistance.
Fig. 10.
The impact of FCPS on glucose consumption in IR-HepG2 cells. (a) Cell survival after 24 h of PA treatment. (b) Glucose uptake during PA treatment. FCPS treatment for 24 h (c) and 36 h (d), respectively. The statistical significance levels for the comparison between PA and untreated groups are as follows: #p < 0.05, ##p < 0.01, ###p < 0.001.*p < 0.05, **p < 0.01, ***p < 0.001, for the comparison between FCPS and PA.
3.9. FCPS promoted DC maturation in vitro
The flow cytometry results indicated that the cell proportion remained unchanged across all groups, suggesting that the viability of DCs was not impacted by the chosen doses of FCPS (Fig. 11a). The CD40 and CD86 expressions were greatly increased by FCPS (Fig. 11b), along with an increase in the expression of IL-6 and IL-12p40 (Fig. 11c). DCs are specialized antigen-presenting cells, which activation and secreted cytokines regulate T cells differentiation [70]. Following the activation of DCs, there was a noted rise in the expression of CD40 and CD86 expression [71]. DCs produce IL6 and IL-12, which stimulate CD8+ T cells and increase cytotoxicity [72], [73]. The result showed that FCPS triggered the immune response by activating DCs. Polysaccharides serve as both scaffolds for cellular structures and biocompatible materials, owing to their adjustable rigidity and functionality, making them extensively employed in drug delivery, tissue engineering, and related applications [74]. Consequently, these findings indicated that FCPS could possess promising potential as immunomodulators and vaccine adjuvants.
Fig. 11.
The impact of FCPS on DCs maturation. (a) Cell proportion. (b) DCs CD86 and CD40 expression. (c) Secretion of cytokines by DCs. ***P < 0.001, LPS, FCPS group compared to the untreated group.
4. Conclusions
In summary, the UAE conditions of FCPS were optimized by conducting ultrasound-assisted extraction four times at a solid–liquid ratio of 1:20 (g/mL) and an ultrasound temperature of 70 °C, resulting in an optimum yield of FCPS was 7.97 %. Analysis of the structure revealed that FCPS exhibited a rough surface and a lamellar structure, primarily composed of uniform concentrated polysaccharide (FCPS1) connected through α-glycosidic and β-glycosidic bonds, It possessed an MW of 4.224 × 104 Da, and encompassed diverse monosaccharides containing glucose, galacturonan, arabinose, galactose, rhamnose, and additional constituents. Research showed that FCPS exhibited significant antioxidant activity, effectively scavenging free radicals, repairing H2O2-induced oxidative damage, and reducing intracellular ROS levels. In addition, FCPS was found to ameliorate PA-induced insulin resistance, enhance glucose consumption and lower blood glucose levels. Furthermore, FCPS activated the immune response through DC activation and up-regulation of IL6 and IL12 expression, indicating immunomodulatory activity. The findings demonstrate that the UAE method is highly efficient in isolating FCPS with biological properties, offering a solid scientific foundation for utilizing FCPS in functional foods and pharmaceuticals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all members of the Immunology team of College of Life Science and Technology for their collaborations. This research was supported by the Natural Science Youth Foundation of Xinjiang Uygur Autonomous Region to Weilan Wang (202004610), the Open Project Fund of Key Laboratory of Xinjiang Uygur Autonomous Region to Weilan Wang (2021D04019) and the Doctoral Start-up Foundation of Xinjiang University to Jinyao Li (BS180222).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106680.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
References
- 1.Arvaniti O.S., Samaras Y., Gatidou G., Thomaidis N.S., Stasinakis A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019;119:244–267. doi: 10.1016/j.foodres.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Yao L., Mo Y., Chen D., Feng T., Song S., Wang H., Sun M. Characterization of key aroma compounds in Xinjiang dried figs (Ficus carica L.) by GC–MS, GC–olfactometry, odor activity values, and sensory analyses. Lwt. 2021;150 [Google Scholar]
- 3.Badgujar S.B., Patel V.V., Bandivdekar A.H., Mahajan R.T. Traditional uses, phytochemistry and pharmacology ofFicus carica: a review. Pharm. Biol. 2014;52:1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 4.Lo Turco V., Potortì A.G., Tropea A., Dugo G., Di Bella G. Element analysis of dried figs (Ficus carica L.) from the Mediterranean areas. J. Food Compos. Anal. 2020;90 [Google Scholar]
- 5.Viuda-Martos M., Barber X., Pérez-Álvarez J.A., Fernández-López J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind. Crop. Prod. 2015;69:472–479. [Google Scholar]
- 6.Slatnar A., Klancar U., Stampar F., Veberic R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011;59:11696–11702. doi: 10.1021/jf202707y. [DOI] [PubMed] [Google Scholar]
- 7.Hajam T.A. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): a review. Chem. Biol. Interact. 2022;368 doi: 10.1016/j.cbi.2022.110237. [DOI] [PubMed] [Google Scholar]
- 8.Rasool I.F., Aziz A., Khalid W., Koraqi H., Siddiqui S.A., Al-Farga A., Lai W.-F., Ali A. Industrial application and health prospective of fig (Ficus carica) by-products. Molecules. 2023;28:960. doi: 10.3390/molecules28030960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Chen Z., Shi H., Yu J., Huang G., Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023;93 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaus Herburger S., Mravec J. Bricks out of the wall: polysaccharide extramural functions. Trends Plant Sci. 2022;27:1231–1241. doi: 10.1016/j.tplants.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes P.A.R., Coimbra M.A. The antioxidant activity of polysaccharides: a structure-function relationship overview. Carbohydr. Polym. 2023;314 doi: 10.1016/j.carbpol.2023.120965. [DOI] [PubMed] [Google Scholar]
- 12.Ying Y., Hao W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: a review. Front. Immunol. 2023;14:1147641. doi: 10.3389/fimmu.2023.1147641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y.P., Li C.Y., Peng X., Zou Y.F., Rise F., Paulsen B.S., Wangensteen H., Inngjerdingen K.T. Polysaccharides from Aconitum carmichaelii leaves: Structure, immunomodulatory and anti-inflammatory activities. Carbohydr. Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119655. [DOI] [PubMed] [Google Scholar]
- 14.Ryu D.H., Cho J.Y., Sadiq N.B., Kim J.C., Lee B., Hamayun M., Lee T.S., Kim H.S., Park S.H., Nho C.W., Kim H.Y. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127645. [DOI] [PubMed] [Google Scholar]
- 15.Yang B., Luo Y., Wu Q., Yang Q., Kan J. Hovenia dulcis polysaccharides: influence of multi-frequency ultrasonic extraction on structure, functional properties, and biological activities. Int. J. Biol. Macromol. 2020;148:1010–1020. doi: 10.1016/j.ijbiomac.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Kamal H., Ali A., Manickam S., Le C.F. Impact of cavitation on the structure and functional quality of extracted protein from food sources – an overview. Food Chem. 2023;407 doi: 10.1016/j.foodchem.2022.135071. [DOI] [PubMed] [Google Scholar]
- 17.Aslam R., Alam M.S., Ali A., Tao Y., Manickam S. A chemometric approach to evaluate the effects of probe-type ultrasonication on the enzyme inactivation and quality attributes of fresh amla juice. Ultrason. Sonochem. 2023;92 doi: 10.1016/j.ultsonch.2022.106268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Zhou X., Sheng L., Zhang D., Zheng X., Pan Y., Yu X., Liang X., Wang Q., Wang B., Li N. Effect of ultrasonic degradation on the structural feature, physicochemical property and bioactivity of plant and microbial polysaccharides: a review. Int. J. Biol. Macromol. 2023;236 doi: 10.1016/j.ijbiomac.2023.123924. [DOI] [PubMed] [Google Scholar]
- 19.Song Z., Xiong X., Huang G. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Zhu J., Wang T., Sun J., Guo T., Zhang L., Yu G., Xia X. Antidiabetic activity of Armillaria mellea polysaccharides: joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochem. 2023;95 doi: 10.1016/j.ultsonch.2023.106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoo D.Y., Low Z.L., Low D.Y.S., Tang S.Y., Manickam S., Tan K.W., Ban Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Yin L.Z., Zhao L., Shu G., Yuan Z.X., Fu H.L., Lv C., Lin J.C. Optimization of the ultrasound-assisted extraction of antioxidant phloridzin from Lithocarpus polystachyus Rehd. using response surface methodology. J. Sep. Sci. 2017;40:4329–4337. doi: 10.1002/jssc.201700686. [DOI] [PubMed] [Google Scholar]
- 23.Long X., Yan Q., Cai L., Li G., Luo X. Box-Behnken design-based optimization for deproteinization of crude polysaccharides in Lycium barbarum berry residue using the Sevag method. Heliyon. 2020;6:e03888. doi: 10.1016/j.heliyon.2020.e03888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan Mei Chin B., Ali A., Kamal H., Mustafa M.A., Khaliq G., Siddiqui Y. Optimizing parameters on the antioxidant capacity of watermelon pulp using conventional orbital shaker and ultrasound-assisted extraction methods. J. Food Process. Preserv. 2020;45:e15123. [Google Scholar]
- 25.Song X., Tang J. Extraction optimization, preliminary characterization and bioactivities in vitro of Ligularia hodgsonii polysaccharides. Int. J. Mol. Sci. 2016;17:788. doi: 10.3390/ijms17050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G., Wang F., Wang M.M., Tang M.T., Zhou T., Gu Q. Physicochemical, structural and rheological properties of pectin isolated from citrus canning processing water. Int. J. Biol. Macromol. 2022;195:12–21. doi: 10.1016/j.ijbiomac.2021.11.203. [DOI] [PubMed] [Google Scholar]
- 27.Aipire A., Yuan P., Aimaier A., Cai S., Mahabati M., Lu J., Ying T., Zhang B., Li J. Preparation characterization, and immuno-enhancing activity of polysaccharides from Glycyrrhiza Uralensis. Biomolecules. 2020;10:159. doi: 10.3390/biom10010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y., Zheng G., You L., Wen L., Li C., Fu X., Zhou L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods. 2017;33:286–296. [Google Scholar]
- 29.Zhou F., Aipire A., Xia L., Halike X., Yuan P., Sulayman M., Wang W., Li J. Marchantia polymorpha L. ethanol extract induces apoptosis in hepatocellular carcinoma cells via intrinsic- and endoplasmic reticulum stress-associated pathways. Chin. Med. 2021;16:94. doi: 10.1186/s13020-021-00504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abudujilile D., Wang W., Aimaier A., Chang L., Dong Y., Wang Y., Fan X., Ma Y., Wang Y., Ziyayiding D., Ma Y., Lv J., Li J. Cistanche tubulosa phenylethanoid glycosides suppressed adipogenesis in 3T3-L1 adipocytes and improved obesity and insulin resistance in high-fat diet induced obese mice. BMC Complement Med. Ther. 2022;22:270. doi: 10.1186/s12906-022-03743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan P., Liu L., Aipire A., Zhao Y., Cai S., Wu L., Yang X., Aimaier A., Lu J., Li J. Evaluation and mechanism of immune enhancement effects of Pleurotus ferulae polysaccharides-gold nanoparticles. Int. J. Biol. Macromol. 2023;227:1015–1026. doi: 10.1016/j.ijbiomac.2022.11.277. [DOI] [PubMed] [Google Scholar]
- 32.Samaram S., Mirhosseini H., Tan C.P., Ghazali H.M., Bordbar S., Serjouie A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015;172:7–17. doi: 10.1016/j.foodchem.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 33.Gu F., Tao L., Chen R., Zhang J., Wu X., Yang M., Sheng J., Tian Y. Ultrasonic-cellulase synergistic extraction of crude polysaccharides from Moringa oleifera leaves and alleviation of insulin resistance in HepG2 Cells. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232012405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Li N., Wang C., Chang T., Jiang H. Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: optimization study. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalmau E., Rosselló C., Eim V., Ratti C., Simal S. Ultrasound-assisted aqueous extraction of biocompounds from orange byproduct: experimental kinetics and modeling. Antioxidants. 2020;9 doi: 10.3390/antiox9040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maran J.P., Priya B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. fruit peel. Int. J. Biol. Macromol. 2014;70:530–536. doi: 10.1016/j.ijbiomac.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 38.Pan G., Yu G., Zhu C., Qiao J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS) Ultrason. Sonochem. 2012;19:486–490. doi: 10.1016/j.ultsonch.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Yin X., You Q., Jiang Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2011;86:1358–1364. doi: 10.1016/j.carbpol.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 40.Teshale F., Narendiran K., Beyan S.M., Srinivasan N.R. Extraction of essential oil from rosemary leaves: optimization by response surface methodology and mathematical modeling. Applied Food Research. 2022;2 [Google Scholar]
- 41.Porter N.T., Martens E.C. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu. Rev. Microbiol. 2017;71:349–369. doi: 10.1146/annurev-micro-102215-095316. [DOI] [PubMed] [Google Scholar]
- 42.Qin W., Sun L., Miao M., Zhang G. Plant-sourced intrinsic dietary fiber: Physical structure and health function. Trends Food Sci. Technol. 2021;118:341–355. [Google Scholar]
- 43.Gong P., Wang S., Liu M., Chen F., Yang W., Chang X., Liu N., Zhao Y., Wang J., Chen X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020;494 doi: 10.1016/j.carres.2020.108037. [DOI] [PubMed] [Google Scholar]
- 44.Ji X., Liu F., Peng Q., Wang M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv. Muzao. Food Chem. 2018;245:1124–1130. doi: 10.1016/j.foodchem.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 45.Molaei H., Jahanbin K. Structural features of a new water-soluble polysaccharide from the gum exudates of Amygdalus scoparia Spach (Zedo gum) Carbohydr. Polym. 2018;182:98–105. doi: 10.1016/j.carbpol.2017.10.099. [DOI] [PubMed] [Google Scholar]
- 46.Liu W., Liu Y., Zhu R., Yu J., Lu W., Pan C., Yao W., Gao X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016;147:114–124. doi: 10.1016/j.carbpol.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., Wang Q., Liu H., Niu L., Li M., Jia Q. A heteropolysaccharide from Rhodiola rosea L.: preparation, purification and anti-tumor activities in H22-bearing mice. Food Sci. Human Wellness. 2023;12:536–545. [Google Scholar]
- 48.Jeong H.K., Lee D., Kim H.P., Baek S.-H. Structure analysis and antioxidant activities of an amylopectin-type polysaccharide isolated from dried fruits of Terminalia chebula. Carbohydr. Polym. 2019;211:100–108. doi: 10.1016/j.carbpol.2019.01.097. [DOI] [PubMed] [Google Scholar]
- 49.Le B., Pham T.N.A., Yang S.H. Prebiotic potential and anti-inflammatory activity of soluble polysaccharides obtained from soybean residue. Foods. 2020;9 doi: 10.3390/foods9121808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P., Liu L., Cheng Z., Zhang Y., Zheng B., Hu X., Zeng H. Structure elucidation and in vitro rat intestinal fermentation properties of a novel sulfated glucogalactan from Porphyra haitanensis. Food Sci. Human Wellness. 2023;12:596–606. [Google Scholar]
- 51.Xie J.-H., Wang Z.-J., Shen M.-Y., Nie S.-P., Gong B., Li H.-S., Zhao Q., Li W.-J., Xie M.-Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016;53:7–15. [Google Scholar]
- 52.Yang T., Jia M., Zhou S., Pan F., Mei Q. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int. J. Biol. Macromol. 2012;50:768–772. doi: 10.1016/j.ijbiomac.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 53.Huang L., Shen M., Morris G.A., Xie J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019;92:1–11. [Google Scholar]
- 54.Jiang Y., Zi W., Pei Z., Liu S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi Pharmaceut. J. 2018;26:656–664. doi: 10.1016/j.jsps.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Huang G., Hu J. Extraction, characterisation and antioxidant activity of polysaccharides from Chinese watermelon. Int. J. Biol. Macromol. 2018;111:1304–1307. doi: 10.1016/j.ijbiomac.2018.01.088. [DOI] [PubMed] [Google Scholar]
- 56.Meng X., Che C., Zhang J., Gong Z., Si M., Yang G., Cao L., Liu J. Structural characterization and immunomodulating activities of polysaccharides from a newly collected wild Morchella sextelata. Int. J. Biol. Macromol. 2019;129:608–614. doi: 10.1016/j.ijbiomac.2019.01.226. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y., Zhang H., Wang Y., Nie S., Li C., Xie M. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2015;186:231–238. doi: 10.1016/j.foodchem.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 58.Yuan L., Qiu Z., Yang Y., Liu C., Zhang R. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 2022;385 doi: 10.1016/j.foodchem.2022.132637. [DOI] [PubMed] [Google Scholar]
- 59.Krol M., Kepinska M. Human nitric oxide synthase-its functions, polymorphisms, and inhibitors in the context of inflammation, diabetes and cardiovascular diseases. Int. J. Mol. Sci. 2020;22 doi: 10.3390/ijms22010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y., Juliet I.C., Wen C., Duan Y., Zhou J., He Y., Zhang H., Ma H. Effects of multi-mode divergent ultrasound pretreatment on the physicochemical and functional properties of polysaccharides from Sagittaria sagittifolia L. Food Biosci. 2021;42 [Google Scholar]
- 61.Vieira Gomes D.C., de Alencar M.V.O.B., dos Reis A.C., de Lima R.M.T., de Oliveira Santos J.V., da Mata A.M.O.F., Soares Dias A.C., da Costa J.S., de Medeiros M.d.G.F., Paz M.F.C.J., Gayoso e Almendra Ibiapina Moreno L.C., Castro e Sousa J.M.d., Islam M.T., Melo Cavalcante A.A.d.C. Antioxidant, anti-inflammatory and cytotoxic/antitumoral bioactives from the phylum Basidiomycota and their possible mechanisms of action. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108643. [DOI] [PubMed] [Google Scholar]
- 62.Li Q., Sun X., Gu G., Guo Z. Novel water soluble chitosan derivatives with 1,2,3-triazolium and their free radical-scavenging activity. Mar. Drugs. 2018;16 doi: 10.3390/md16040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galano A., Mazzone G., Alvarez-Diduk R., Marino T., Alvarez-Idaboy J.R., Russo N. Food antioxidants: chemical insights at the molecular level. Ann. Rev. Food Sci. Technol. 2016;7:335–352. doi: 10.1146/annurev-food-041715-033206. [DOI] [PubMed] [Google Scholar]
- 64.Lam P.L., Wong R.S., Lam K.H., Hung L.K., Wong M.M., Yung L.H., Ho Y.W., Wong W.Y., Hau D.K., Gambari R., Chui C.H. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem. Biol. Interact. 2020;320 doi: 10.1016/j.cbi.2020.109023. [DOI] [PubMed] [Google Scholar]
- 65.Halliwell B., Adhikary A., Dingfelder M., Dizdaroglu M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021;50:8355–8360. doi: 10.1039/d1cs00044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hao L., Gao X., Zhou T., Cao J., Sun Y., Dang Y., Pan D. Angiotensin I-converting enzyme (ACE) inhibitory and antioxidant activity of umami peptides after in vitro gastrointestinal digestion. J. Agric. Food Chem. 2020;68:8232–8241. doi: 10.1021/acs.jafc.0c02797. [DOI] [PubMed] [Google Scholar]
- 67.Skouta R., Moran-Santibanez K., Valenzuela C.A., Vasquez A.H., Fenelon K. Assessing the antioxidant properties of larrea tridentata extract as a potential molecular therapy against oxidative stress. Molecules. 2018;23 doi: 10.3390/molecules23071826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bao T., Karim N., Ke H., Tangpong J., Chen W. Polysaccharide isolated from wax apple suppresses ethyl carbamate-induced oxidative damage in human hepatocytes. J. Zhejiang Univ.-Sci. B. 2023;24:574–586. doi: 10.1631/jzus.B2200629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallet M.A., Sen P., Tisch R. Immunoregulation of dendritic cells. Clin. Med. Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Racioppi L., Cancrini C., Romiti M.L., Angelini F., Di Cesare S., Bertini E., Livadiotti S., Gambarara M.G., Matarese G., Lago Paz F., Stefanini M., Rossi P. Defective dendritic cell maturation in a child with nucleotide excision repair deficiency and CD4 lymphopenia. Clin. Exp. Immunol. 2001;126:511–518. doi: 10.1046/j.1365-2249.2001.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalyanasundaram Bhanumathy K., Zhang B., Xie Y., Xu A., Tan X., Xiang J. Potent immunotherapy against well-established thymoma using adoptively transferred transgene IL-6-engineered dendritic cell-stimulated CD8+ T-cells with prolonged survival and enhanced cytotoxicity. J. Gene Med. 2015;17:153–160. doi: 10.1002/jgm.2836. [DOI] [PubMed] [Google Scholar]
- 73.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 74.Su L., Feng Y., Wei K., Xu X., Liu R., Chen G. Carbohydrate-based macromolecular biomaterials. Chem. Rev. 2021;121:10950–11029. doi: 10.1021/acs.chemrev.0c01338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.