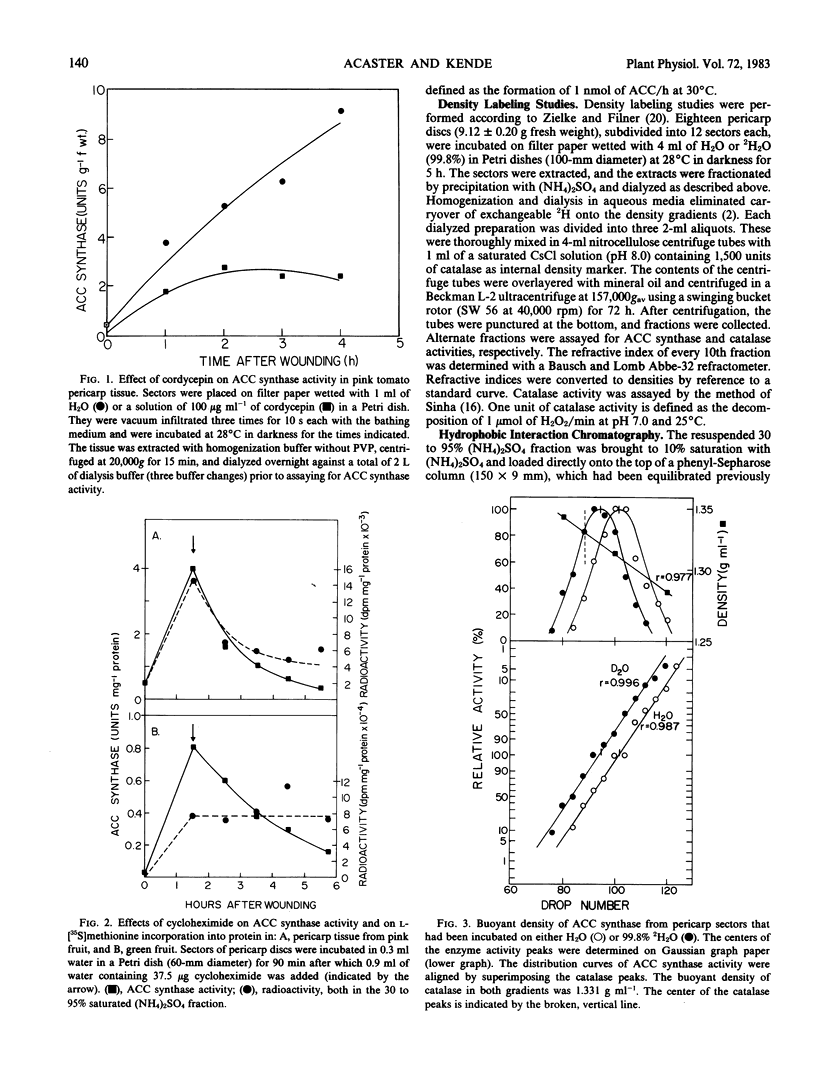
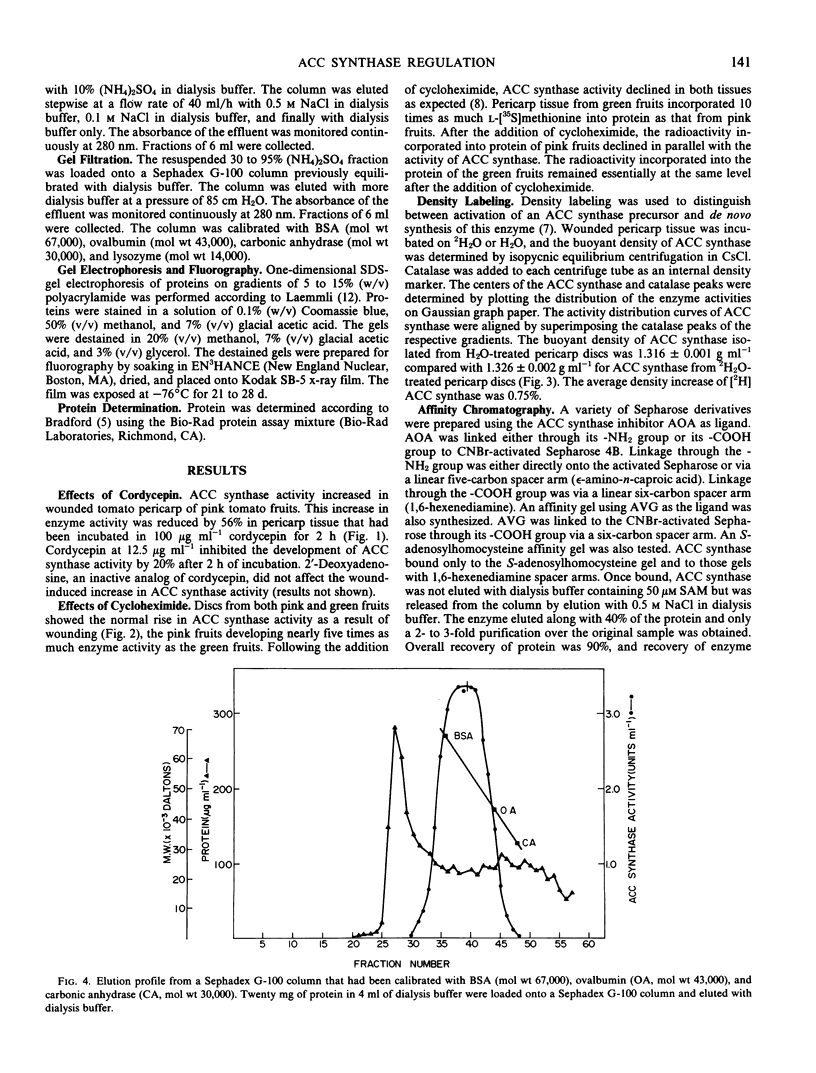
Abstract
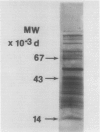
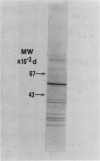
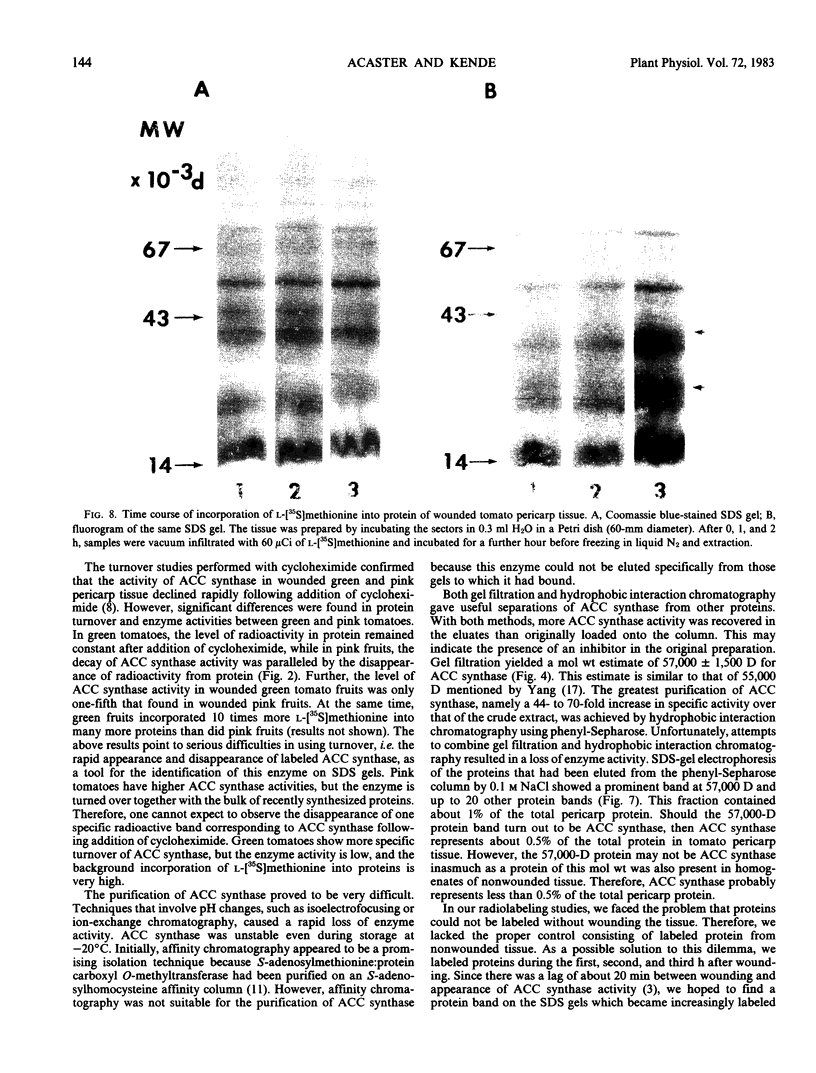
We studied the regulation of 1-aminocyclopropane-1-carboxylate (ACC) synthase activity in tomato (Lycopersicon esculentum Mill.) fruit tissue and attempted the purification of this enzyme. The increase of ACC synthase activity in wounded tomato pericarp was inhibited by cordycepin and cycloheximide. Density labeling studies showed a 0.75% increase in the buoyant density of ACC synthase isolated from tomato pericarp tissue that had been incubated on 2H2O as compared to ACC synthase from H2O-treated tissue. These data are consistent with the hypothesis that ACC synthase is synthesized de novo following wounding of tomato pericarp tissue. SDS-gel electrophoresis and fluorography showed that the pattern of incorporation of l-[35S]methionine into protein changed with time after wounding of the tissue. Radioactive protein bands that were not detected 1 hour after wounding, became apparent 2 to 3 hours after wounding.
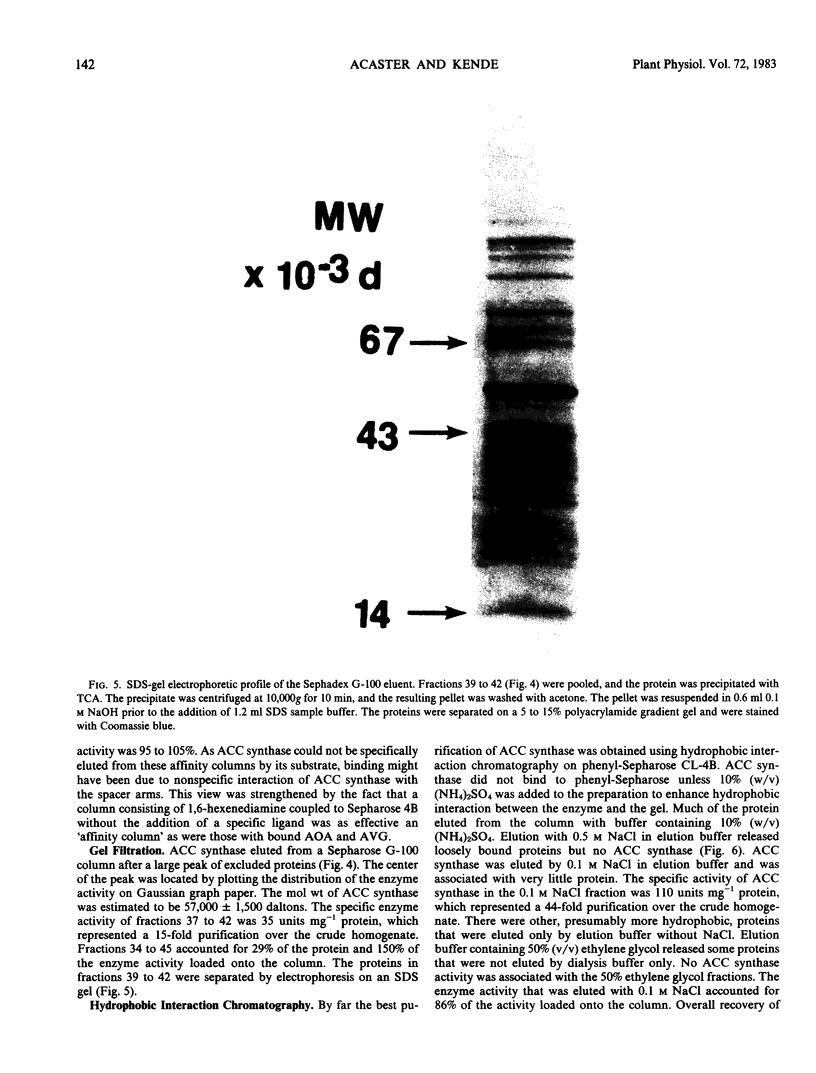
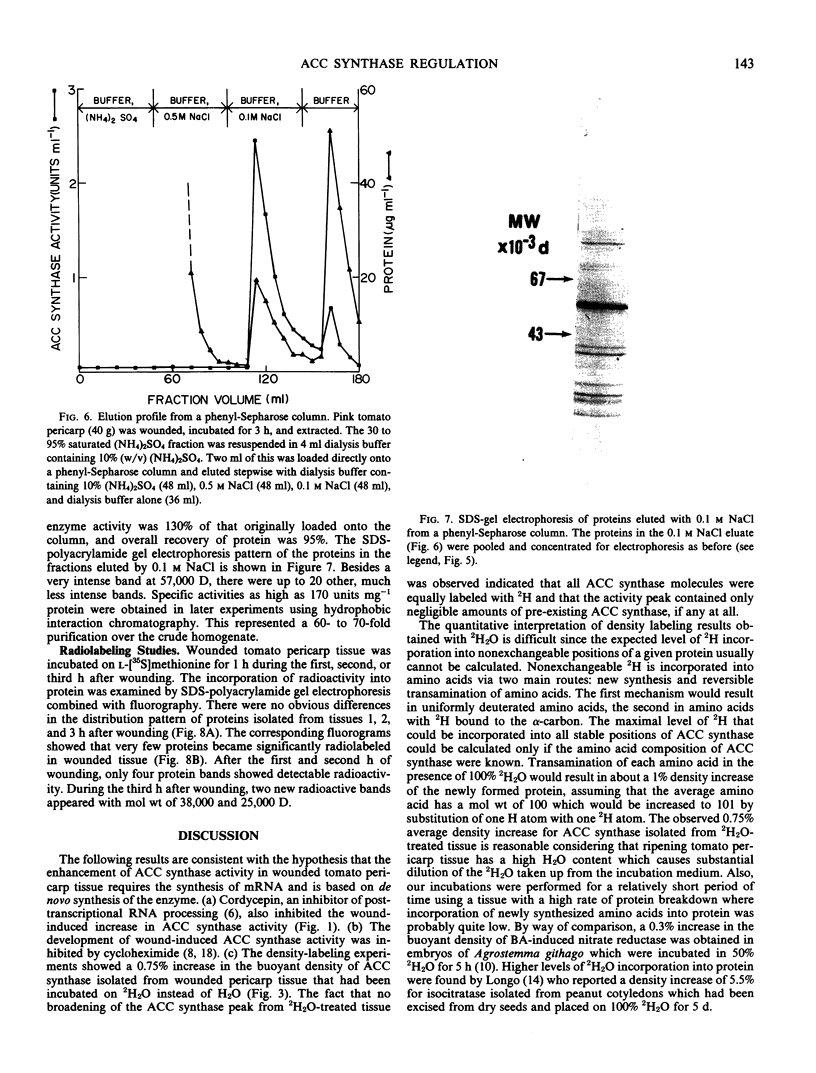
Gel filtration on Sephadex G-100 gave a molecular weight estimate for ACC synthase of 57,000 ± 1,500 daltons. Hydrophobic interaction chromatography on phenyl-Sepharose yielded a 60- to 70-fold purification of the enzyme. SDS-gel electrophoresis of this preparation indicated the presence of one intense band at 57,000 daltons and several less intense bands. Affinity chromatography was of limited usefulness in the purification of ACC synthase since the enzyme could not be eluted specifically from any of the affinity gels tried. Purification methods that involved pH changes led to a rapid loss of ACC synthase activity. ACC synthase was estimated to comprise less than 1% of the total protein in tomato pericarp tissue.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstine W., Jacobsen J. V., Scandalios J. G., Varner J. E. Deuterium oxide as a density label of peroxidases in germinating barley embryos. Plant Physiol. 1970 Feb;45(2):148–152. doi: 10.1104/pp.45.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Shen T. C. Nitrate reductase in Agrostemma githago. Comparison of the inductive effects of nitrate and cytokinin. Biochim Biophys Acta. 1972 Nov 24;286(1):118–125. doi: 10.1016/0304-4165(72)90097-9. [DOI] [PubMed] [Google Scholar]
- Kim S., Nochumson S., Chin W., Paik W. K. A rapid method for the purification of S-adenosylmethionine: protein-carboxyl O-methyltransferase by affinity chromatography. Anal Biochem. 1978 Feb;84(2):415–422. doi: 10.1016/0003-2697(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. K. Colorimetric assay of catalase. Anal Biochem. 1972 Jun;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Biosynthesis of wound ethylene. Plant Physiol. 1980 Aug;66(2):281–285. doi: 10.1104/pp.66.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]