Abstract
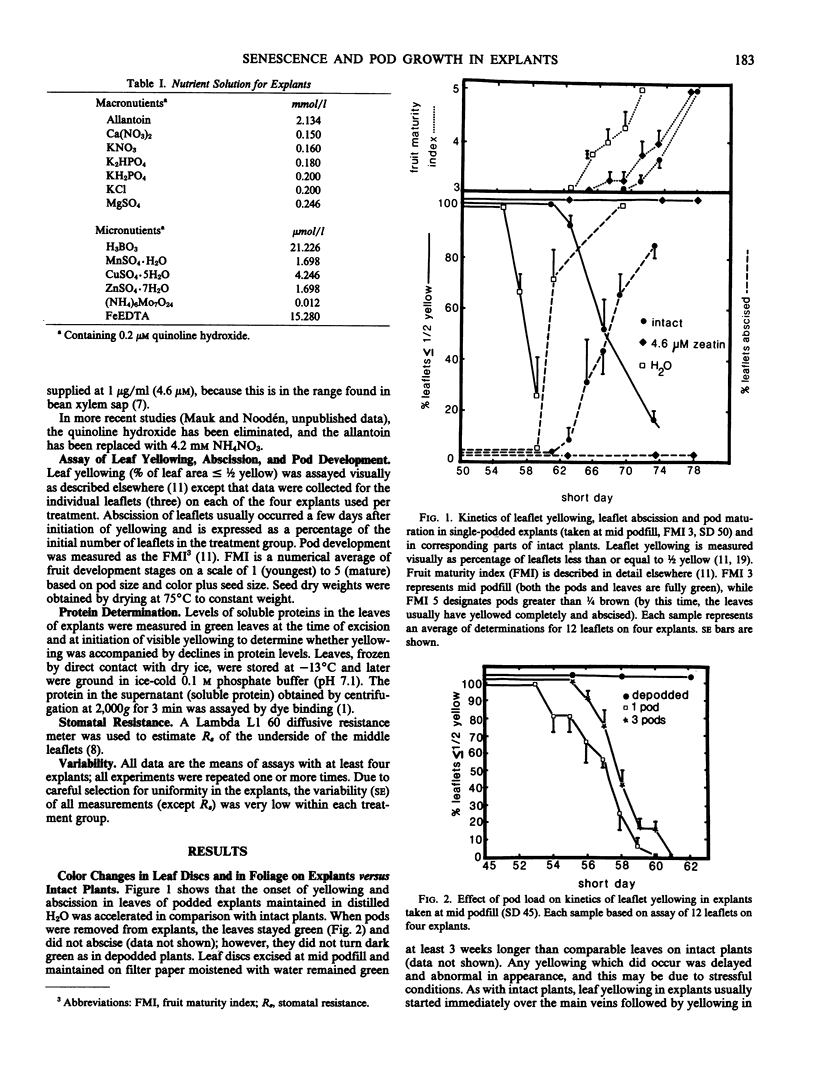
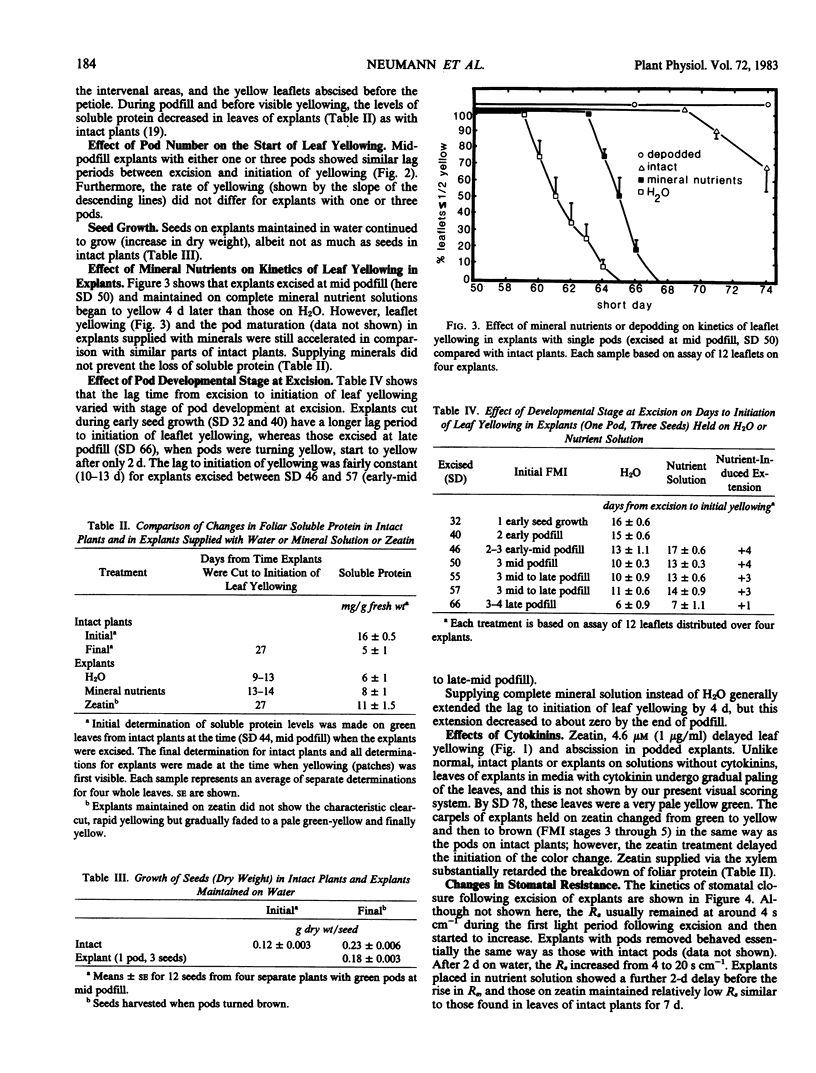
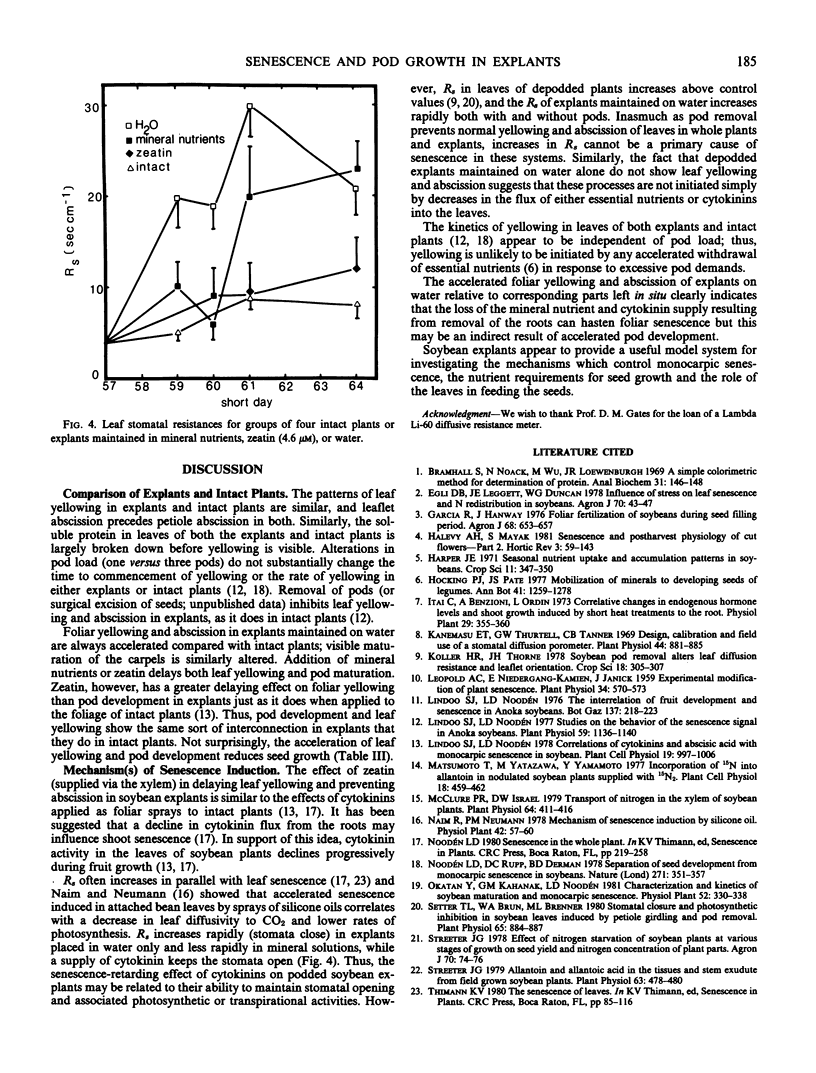
Excised soybean (Glycine max [L.] Merrill) cv Anoka leaf discs tend to remain green even after the corresponding intact leaves have turned yello on fruiting plants. We have found that explants which include a leaf along with a stem segment (below the node) and one or more pods (maintained on distilled H2O) show similar but accelerated leaf yellowing and abscission compared with intact plants. In podded explants excised at pre-podfill, the leaves begin to yellow after 16 days, whereas those excised at late podfill begin to yellow after only 6 days. Although stomatal resistances remain low during the first light period after excision, they subsequently increase to levels above those in leaves of intact plants. Explants taken at mid to late podfill with one or more pods per node behave like intact plants in that pod load does not affect the time lag to leaf yellowing. Explant leaf yellowing and abscission are delayed by removal of the pods or seeds or by incubation in complete mineral nutrient solution or in 4.6 micromolar zeatin. Like chorophyll breakdown, protein loss is accelerated in the explants, but minerals or especially zeatin can retard the loss. Pods on explants show rates and patterns of color change (green to yellow to brown) similar to those of pods on intact plants. These changes start earlier in explants on water than in intact plants, but they can be delayed by adding zeatin. Seed dry weight increased in explants, almost as much as in intact plants. Explants appear to be good analogs of the corresponding parts of the intact plant, and they should prove useful for analyzing pod development and mechanisms of foliar senescence. Moreover, our data suggest that the flux of minerals and cytokinin from the roots could influence foliar senescence in soybeans, but increased stomatal resistance does not seem to cause foliar senescence.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Kanemasu E. T., Thurtell G. W., Tanner C. B. Design calibration and field use of a stomatal diffusion porometer. Plant Physiol. 1969 Jun;44(6):881–885. doi: 10.1104/pp.44.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Niedergang-Kamien E., Janick J. Experimental Modification of Plant Senescence. Plant Physiol. 1959 Sep;34(5):570–573. doi: 10.1104/pp.34.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Stomatal closure and photosynthetic inhibition in soybean leaves induced by petiole girdling and pod removal. Plant Physiol. 1980 May;65(5):884–887. doi: 10.1104/pp.65.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


