Abstract
Objective
The study objective was to determine the effects a high protein (HP) vs. a high carbohydrate (HC) diet on cardiovascular risk factors (CVR), inflammation, metabolic parameters, oxidative stress, weight loss, lean and fat body mass, and remission of Type 2 Diabetes (T2DM) in subjects with obesity.
Research design and methods
Twelve women and men with T2D were recruited and randomized to either a HP (30%protein, 30%fat, 40%carbohydrate) (n = 6) or HC (15%protein, 30%fat, 55%carbohydrate) (n = 6) diet feeding study for 6 months in this randomized controlled trial. All meals were purchased at local grocery stores and provided to subjects for 6 months with daily food menus for HP or HC compliance with weekly food pick-up and weight measurements. Oral glucose tolerance and meal tolerance tests with glucose and insulin measurements and DXA scans were done at baseline and after 6 months on the respective diets.
Results
After 6 months on the HP diet, 100% of the subjects had remission of their T2DM to Normal Glucose Tolerance (NGT), whereas only 16.6% of subjects on the HC diet had remission of their T2DM. The HP diet group exhibited significant improvement in a) cardiovascular risk factors (p = 0.004, b) inflammatory cytokines(p = 0.001), c) insulin sensitivity(p = 0.001), d) oxidative stress(p = 0.001), e) increased %lean body mass(p = 0.001) compared to the HC diet group at 6 months.
Conclusions
A significant improvement in cardiovascular risk factors, inflammation, metabolic parameters and 100% remission of T2DM to NGT was achieved with a HP diet compared to a HC diet at 6 months.
Clinicaltrials.gov. identifier
Keywords: Cardiovascular risk factors, Inflammation, Weight loss, Type 2 diabetes remission, Insulin sensitivity, High protein diet
1. Introduction
The prevalence of people with Type 2 Diabetes Mellitus (T2DM) has continued to increase over the past 20 years with currently approximately 37 million people with diabetes, 27 million who are diagnosed and 7.2 million who are undiagnosed in the USA according to the Center for Disease Control(CDC) 2022 National Diabetes Statistics Report [1]. Obesity is one of the highest risk factors for T2DM, heart disease, hypertension, and other metabolic disease [2] and has reached epidemic proportion in the U.S. with over 42% adults estimated to be obese [3,4]. As BMI increases from 23 to >35 kg/m2 there is 93 fold increase in T2DM [4,5]. The Diabetes Prevention Program (DPP) [6,7] and ACT NOW [8] studies showed that the rate of conversion for Impaired Glucose Tolerance (IGT) (prediabetes) to T2DM is between 7 and 10%/year with no significant difference in ethnicity. Subjects with diabetes are at increased risk of numerous medical issues and complications such as those seen in COVID-19 subjects with diabetes who have had a higher incidence of hospitalization and mortality [[9], [10], [11]]. DPP [6,12] and other similar diabetes prevention studies [13,14] have shown the importance of diet in reducing the risk of conversion of IGT to T2DM. Attempts to reduce the risk of T2DM, complications and medical costs must start in the early part of diagnosis [[15], [16], [17]]. Although numerous diets have been recommended for T2DM [[18], [19], [20], [21], [22]] and non-diabetics, while some studies suggested advantages of low-carbohydrate diet [23,24] and other studies suggested advantages of a high-protein diet [25,26], there is no consensus on a specific weight loss diet to manage blood glucose in T2DM [27]. Also, there has not been established a diet for weight loss and glucose control and converting from T2DM to Normal Glucose Tolerance (NGT). Given the metabolic changes that occur with T2DM it seems prudent to determine if one exists.
Our studies of the effect of macronutrients with a High Protein (HP) diet or High Carbohydrate (HC) diet on IGT subjects with obesity [28] and NGT subjects with obesity [29] demonstrated similar weight loss of 9–10% in both diet interventions, but greater advantages of the HP diet for insulin sensitivity (100% remission of IGT to NGT), CVR factors, oxidative stress (ROS), inflammatory cytokines (IC) [28,29]. HP intake has the potential to suppress hunger and induce satiety [18,25,30] with a negative relationship between protein content and glycemic index [31,32]. Increased protein intake has also been shown to reduce energy intake independent of the effect of satiety [25]. Proteins also increase the thermic effect of feeding [33] mostly by increasing protein synthesis. Elevated levels of lipids are considered a primary risk factor for CV disease and dietary composition can affect the lipid levels [[34], [35], [36], [37]]. Our studies showed a greater decrease in TG in the HP diet [28,29] demonstrating that increasing proteins in the diet may alter the lipid profile in a beneficial way. It has been shown that protein intake by itself induces insulin release, is different in diabetic and non-diabetic individuals [38] and protein is a much less potent secretagogue for insulin than is glucose in normal individuals [39]. Our studies showed a lower insulin response to the HP than the HC diet [28,29]. This suggests that HP diets may help preserve the Beta cells by increasing sensitivity and decreasing insulin load per meal. Our studies on the incretin response to HP and HC diets showed an increased release in GLP-1 and GIP with the HP diet compared to the HC diet [40,41]. Treatment of T2DM subjects with GLP-1 receptor agonists and DPP-4 inhibitors have been found to improve insulin sensitivity and cardiovascular risks [27,[42], [43], [44], [45], [46], [47], [48]]. These studies suggest that our HP diet may be beneficial in treating T2DM and have cardiovascular benefits.
Another important aspect of the HP diet is the decreased glucose area under the curve (AUC) and anti-inflammatory effect compared to the HC diet [28,29]. Studies have demonstrated that hyperglycemia or elevation of Free Fatty Acids (FFA) leads to activation of leukocytes and increase in cytokines and reactive oxygen species (ROS) [[49], [50], [51], [52]]. Studies have shown that hyperglycemia in T2DM and obesity is associated with increased inflammatory cytokines [53,54]. Thus, reduction in inflammatory cytokines in diabetes is important in leu of the fact that patients that develop acute respiratory distress [[55], [56], [57], [58]] and COVID-19 have increased inflammatory cytokines and diabetes would add to this inflammatory effect [59].
Protein content in the diet may also help maintain lean body mass [28,60,61]. Few studies have compared diets with adequate percentages of macronutrients and sufficient follow-up time where high protein is compared to high carbohydrate diets and determined effects on CVR factors, ROS, inflammatory cytokines [28,29,52,53,[62], [63], [64], [65], [66]] especially with respect to subjects with Type 2 Diabetes.
In deference to all other diet studies, in this study we determined the remission of type 2 diabetes in newly diagnosed type 2 diabetes subjects with obesity and determined longitudinally the changes in various metabolic markers of insulin sensitivity, inflammatory cytokine, CVR factors, ROS, and change in muscle and fat mass, with our tightly controlled HP and HC diets weight loss feeding study from baseline to 6 months. These studies showed the significant impact of the HP diet on these parameters in T2DM and remission.
2. Research design and methods
2.1. Patients
Women and men subjects age 20–60 years old with a BMI ≥30 kg/m2 to ≤55 kg/m2 diagnosed with type 2 diabetes with the past two years or diagnosed at this study screening visit were recruited for the study. Subjects were selected on the basis of inclusion criteria of age, BMI, fasting glucose of ≥126 mg/dl, 2 h glucose level of >200 mg/dl during a standard oral glucose tolerance test, and HbA1c of 6.5–10%. They were excluded if they had proteinuria or elevated serum creatinine(>1.5 mg/dl), history of liver disease, abnormal liver function tests, on antidiabetic agents or insulin, thyroid disease with abnormal TSH, weight >350 lbs, triglycerides >400 mg/dl, LDL cholesterol >160 mg/dl, SBP >145 or DBP >100 mm, use of medications known to effect glucose or lipid metabolism, pregnancy or the desire to become pregnant in the next 6 months, weight loss of more than 5% of body weight in the last 6 months, smoked, or history of cancer undergoing active treatment. Subjects that were on metformin who wanted to participate in the study were discontinued from the drug by their PCP and baseline tests were performed on these subjects 4 weeks after discontinuing the drug. Subjects that met the above criteria were asked to keep a food diary for a week. Those found to be non-adherent and unable to keep diet diary or deemed unable to adhere to the protocol were excluded from the study.
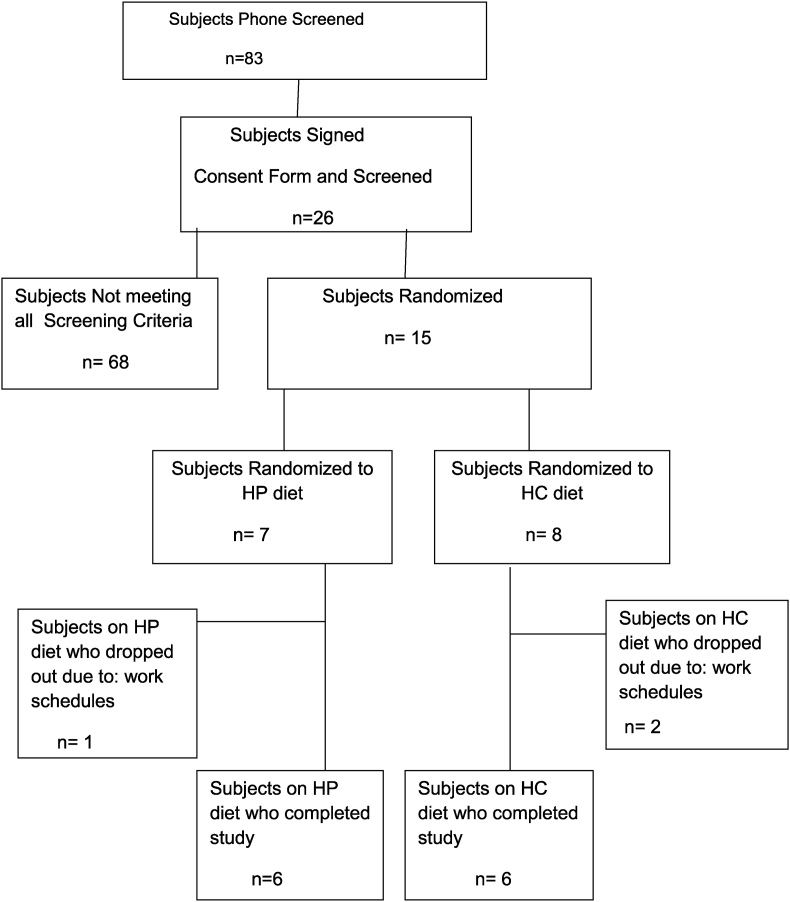
Of the 83 subjects screened by phone 26 were asked to come in to sign the consent form and testing for meeting the inclusion criteria. Fifteen of these subjects met all the inclusion criteria and were randomized to a HP diet (7 subject) vs. HC diet (8 subjects) for a period of 6 months. One subject in the HP group and 2 subjects in the HC group dropped out within a few weeks after screening due to their work schedule, driving distance or moved out of area. Since there was no data (OGTT, MTT, DXA, weight, metabolic markers) other than baseline on those subjects that dropped out they were not included in the data analysis of comparison of changes from baseline to 6 months on the diet interventions. Therefore, six subjects in each group completed the 6 month study as shown in Fig. 1 and data analyzed.
Fig. 1.
Shows the recruiting and screening of subjects for the participants in the study.
2.2. Study design
The study was a prospective randomized trial of a High Protein (HP) diet (30% Kcals from protein, 40% Kcals from carbohydrate (CHO), 30% Kcals from fat) vs High Carbohydrate (HC) diet (15% Kcals from protein, 55% Kcals from CHO, 30% Kcals from fat) for a period of 6 months. The study was approved by the Institutional Review Board of the University of Tennessee Health Science Center (UTHSC).
All participants were seen in the General Clinical Research Center (GCRC) at UTHSC for all their visits. After signing the consent form, a history and physical examination, height and weight, blood pressure and waist measurements were done. At the baseline and at 6 months of the study participants underwent a standard OGTT and mixed meal tolerance test (MMT). The meal for the MTT for the HP group was a high protein meal and the meal for the MTT for the HC group was a HC meal. Both HP and HC meals were 300 calories (the same as the 75 gm OGTT). Each provocative test was done after an overnight fast with two or more days between tests. Glucose and insulin were measured at baseline and at 30 min intervals for 2 h. These tests were repeated after being on the diet for 6 months. Dual energy x-ray absorptiometry (DXA) scan, resting metabolic rate (RMR), chemistry profile, Complete Blood Count(CBC), vitamin D, Parathyroid Hormone(PTH), lipid profiles, as well as 24 h urine collections for creatinine clearance (CrCl), microalbumin, calcium(Ca) and urinary urea nitrogen (UUN) were all done at baseline and 6 months. These determinations were done to determine insulin sensitivity and glucose response, lipid profile, changes in body weight and body composition (lean and fat mass), calcium metabolism and protein breakdown (by urinalysis). Subjects were assessed for level of activity and all were at minimum activity. Since the American Diabetes Association [27] recommends 150 min of exercise per week patients were asked to walk 30 min a day and were given FitBits to monitor their level of physical activity which was monitored at a weekly level throughout the study. After meeting the screening criteria subjects were randomized to either the HP or HC diet using a permuted block randomization method generated by the biostatistician.
Subjects were considered to have remission of their Type 2 Diabetes to normal glucose tolerance (NGT) if at 6 months they had a fasting glucose of <100 mg/dl, and a 2 h glucose level of <140 mg/dl during a single oral glucose tolerance test and HbA1c ≤ 5.7%. They were considered to have remission to prediabetes if at 6 months they had a fasting glucose of 100 to <126 mg/dl, and a 2 h glucose level of 140–199 mg/dl during the OGTT and HbA1c > 5.7–6.4%. Subjects who at the 6 month OGTT had a fasting glucose of ≥126 mg/dl and a 2 h glucose level of ≥200 mg/dl and HbA1c ≥ 6.5% did not have remission of their Type 2 Diabetes and were referred back to their Primary Care Physician (PCP) or Endocrinologist for pharmaceutical treatment. Subjects who had remission of Type 2 Diabetes were transitioned to purchasing their own food and meal preparations using the diet plans they were using during the study when all food was provided. These subjects were followed for an additional 6 months by phone, emails and dietary consultation to help them maintain their remission of diabetes and weight loss.
3. Diet related paremeters
3.1. Diet composition
Study participants were randomized using a randomization table to either the HP or HC diet for 6 months. Calorie maintenance needs were determined on an individual basis using their resting metabolic rate (RMR). After maintenance caloric needs were established for each individual, 500 Kcals/day were subtracted from the determined caloric needs to promote a 1–2 lbs weekly weight loss. On average, a 1700 Kcal/day diet for a 100 kg individual was used to achieve adequate weight loss. A one-time calorie reduction of 200 Kcal was made if a subject reached a plateau and did not lose weight for two consecutive weeks during the 6 months. No subject was on less than 1200 Kcal/day.
A feeding study where all food and daily menus were provided was necessary to ensure accurate macronutrient consumption. The meals were dispensed as pre-packaged foods from stored frozen food by the dietician affiliated with the UT CRC dietary services on the site of the CRC, maintaining the macronutrient and caloric requirements established at randomization. Subjects were given 3 meals a day plus snacks between meals. All foods were available at local grocery stores and met the recommended daily intake of vitamins and mineral for women and men age 20–60 years as assessed by the University of Minnesota Nutrition Data system. Both the HP and HC diets contained more than the recommended daily intake (RDI) for calcium of 1000 mg/day for women and men 20–60 years of age [67] by providing an average of 1500 mg Ca for both diets. As the participants were obese with Type 2 Diabetes and might be at risk for coronary heart disease, it was prudent to ensure the participants follow a healthy diet that could minimize their health risks. For example, dietary fat sources focused on monounsaturated and polyunsaturated fats, i.e., plant oils, semi-liquid margarine, nuts [63,68,69]. Dietary carbohydrate sources emphasized whole grains, fruits, vegetables and legumes; and dietary protein sources included lean meats, fish, chicken, eggs and non-fat dairy foods, i.e. fat-free milk and low-fat cheese. The dietary principles are consistent with the guidelines of the Institute of Medicine [68] and American Diabetes Association [27].
3.2. Diet compliance
Participants for this feeding study were given detailed instructions during the pre-study orientation at screening visits. A variety of foods with the same macronutrients were offered to increase choices and adherence. After choosing their preferences for meals and snacks, participants were instructed to adhere to the diet to which they were assigned. All food was provided for the entire 6 months to the participants who came in weekly to pick up their food and daily menu food record for the week and weight measurements. Participants were required to turn in their record when they returned for the next food pick up to check for compliance of diet adherence.
Frequent interaction, behavior modification, individualized diet with food variety and food record systems which have been shown to increase dietary compliance were used. Food records served as a “motivational enforcement” and recording dietary intake emphasized the importance of the diet as a key component to the study [70]. Compliance was assessed by objective and subjective parameters which included weekly subject contact and detailed review of their food diaries.
3.3. Assessment of body composition by DXA
Hologic Discovery QDR Bone Densitometer (version 8.3) was used to measure body-composition by measurements of the entire body. DXA measurements were done at baseline and 6 months. Body composition including lean mass (LM), fat mass (FM), and bone mineral content (BMD) were assessed [28]. The DXA Quality Assurance manual for the UTHSC Clinical Research Center (CRC) was used to standardize patient positioning and scan analysis.
3.4. Resting metabolic rate (RMR)
Indirect calorimetry was used to assess RMR of each participant at the beginning of the study to determine the caloric diet intake needs for weight loss for each subject and at 6 months to determine changes in their metabolic rate. A Cardio Coach (Korr Medical Technologies) was used for the determination of the RMR. The subjects after an overnight (10 h) fast were at absolute rest in a reclining position for 15 min after which respiratory exchanges were measured continuously for 15 min measuring oxygen consumption and CO2 production to determine the RMR [28,71].
4. Laboratory procedures
4.1. Determination of plasma metabolic hormones, cytokines, markers of cardiovascular risks and lipids
Glucose and insulin levels for the OGTT and MTT for baseline and 6 months, were measured at 0, 30, 60, 90, and 120 min and AUC calculated using the Trapezoidal rule. Insulin, glucose, inflammatory cytokines (TNFa, IL-1β, IL-6, INFƔ, MCP-1), CVR factors (blood pressure, triglycerides, LDL, HDL, cholesterol, BMI, hcCRP, FFA), adiponectin, oxidative stress (ROS) (dichlorofluorescein (DCF) and MDA), β-hydroxybutyrate, and HbA1c were measured using our previously established methods [28,29,53,57,58,72]. The Coefficient of Variation of the assays were all less than 5%.
Chemistry Metabolic Profile (CMP), CBC with diff, TSH, cortisol, UUN and other tests to exclude chemical and metabolic abnormalities and protein balance were determined by standard clinical lab procedures. Protein and muscle mass catabolism were assessed via 24-h UUN, CrCl and Ca at baseline and at 6 months. Calcium balance was assessed by 24-h urine Ca excretion as well as serum Ca, PTH, and 25 OH-vitamin D at baseline and 6 months. BMI and waist were measured by standard methods.
4.2. Insulin sensitivity and beta cell function
The homeostasis model assessment was used to determine the insulin resistance HOMA IR [73]. Insulin sensitivity (ISI) was determined from plasma insulin and glucose measurements from the OGTT by the Matsuda insulin index [8,74]. Beta cell function were calculated as previously described from plasma glucose and insulin measurements obtained during the 2 h OGTT [8,28,29].
5. Statistical analysis
The primary outcomes examined were remission of Type 2 Diabetes, markers of insulin sensitivity, inflammatory cytokines, cardiovascular risk factors, and change in lean and fat body mass from baseline to 6 months. Initially, change was compared between the two arms using Wilcoxon Rank Sum test to compare the effects of the two diets. In addition, Wilcoxon Signed Rank test was used to compare baseline and 6 months data to assess effects of each diet.
To assess the effectiveness of randomization, Wilcoxon Rank Sum test was used to compare baseline variables between the two arms. A p-value less than 0.05 was considered statistically significant. If important baseline differences were identified, they were included in analysis using generalized linear models.
All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC). Results are presented as mean ± SE. Statistical significance was declared if the two-sided p-value was less than 0.05. The study was designed to recruit 6 subjects in each arm. Power analysis was done according to two scenarios at 5% significance level. In the first objective, the changes were compared between the two arms. Assuming the baseline to follow-up correlation is 0.25 and the effect size (the ratio of variance of interaction effects to within cell variance) is 0.40, interaction effects could be tested with at least 80% power. Just as important, in the absence of interaction effects, focus was shifted on assessing marginal treatment arm differences. Assuming again the baseline-to follow-up correlation is 0.25 and an effect size of 0.80 (ratio of mean weight change to within SD), the changes could be tested with at least 80% power. Note that extended models having baseline covariates should lead to greater statistical power under both scenarios.
6. Results
At baseline the HP and HC groups were not statistically different. Table 1 shows the mean ± SE of various parameters monitored on the twelve (6 HP and 6 HC) diet subjects from baseline through 6 months and the significant difference of changes of the parameters in the subjects on the HP and HC diets. Significantly important is the 100% (6/6) remission of Type 2 Diabetes to normal glucose tolerance (NGT) in all the HP diet group subjects; whereas, there was only a 16% (1/6) remission to NGT and (1/6) remission to IGT in the HC group. Subjects on both the HP and HC had significant weight loss at 6 months from their baseline weights but not significantly different between the HP and HC groups at 6 months. The waist measurements decreased in the HP diet group and in HC diet group decreased from baseline to 6 months. HbA1c was significantly improved to normal range with the HP group whereas, the average HbA1c did not decrease below the criteria level of T2D in the HC diet group after 6 months. Insulin sensitivity (HOMA IR and ISI) and Beta cell function as shown in Table 1 improved significantly from baseline to 6 months in the HP diet group and in the HC group, however, the HP group had significantly greater improvement in these parameters compared to the HC group at 6 months (p = 0.001). Diet compliance was 94.7% and 94.1% for the HP and HC diet groups, respectively, which were not significantly different. Table 2 shows the inflammation markers (TNFα, IL-1β, IL-6, IFNƔ, MCP-1, hsCRP) and CVR factors (BP, cholesterol, triglycerides, LDL, HDL), and oxidative stress (ROS-DCF, MDA) were significantly decreased in both diet groups. The FFA were significantly decreased in the HP diet group at 6 months but not in the HC diet group. The HP diet resulted in significantly greater reduction in these inflammatory cytokines, CVR factors and oxidative stress compared to the HC diet at 6 months. This reduction in TNFα, IL-1β, IL-6, IFNƔ, MCP-1, and hsCRP demonstrates a better anti-inflammatory effect of the HP diet compared to the HC diet.
Table 1.
Changes in insulin sensitivity and metabolic parameters with the HP and HC diets.
| HP (n = 6) | HC (n = 6) | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Baseline | 6 months | pa | Baseline | 6 months | pa | pb |
| Female/Male | 4/2 | 4/2 | |||||
| Ethnicity AA/C | 3/3 | 3/3 | |||||
| BMI (kg/m2) | 39 ± 1.8 | 36 ± 1.9 | <0.001 | 36 ± 1.7 | 33 ± 1.6 | 0.002 | 0.391 |
| WeightLoss(lbs) | 15.4 ± 2.5 | <0.001 | 19.5 ± 2.2 | <0.001 | 0.692 | ||
| Waist (cm) | 112.5 ± 3 | 105.2 ± 2 | 0.001 | 110.2 ± 3.4 | 103.5 ± 4 | 0.01 | 0.0 4 |
| HbA1c | 7.7 ± .05 | 5.6 ± .02 | 0.001 | 7.8 ± .04 | 6.7 ± .06 | 0.01 | 0.002 |
| Insulin Sensitivity | |||||||
| HOMA IR | 5.3 ± 0.29 | 2.1 ± 0.13 | 0.0001 | 5.2 ± 0.27 | 4.4 ± 0.26 | 0.03 | 0.004 |
| ISI(Matsuda I) | 1.4 ± 0.2 | 6.4 ± 0.9 | 0.0001 | 1.5 ± 0.3 | 3.0 ± 0.4 | 0.04 | 0.0001 |
| β Cell Function | 3.1 ± 0.3 | 11.1 ± 2.1 | 0.001 | 3.2 ± 0.3 | 5.26 ± 0.9 | 0.03 | 0.001 |
| % Remission of Type 2 Diabetes | 100% remission to NGT | 16.6% remission to NGT | 0.001 | ||||
ANALYSIS Mean ± SE were calculated.
Wilcoxon Signed Rank Test to compare baseline and 6 mo within diet group. p ≤ 0.05 was considered statistically significant.
Wilcoxon Rank Sum Test was used to compare variables between the two diet groups at 6 mo and.
Table 2.
Changes in inflammatory, CVR and oxidative stress markers with the HP and HC diet.
| Parameters | HP (n = 6) |
HC (n = 6) |
pb | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | pa | Baseline | 6 months | pa | ||
| Inflammation | |||||||
| TNFα (pg/ml) | 19.03 ± 3.53 | 3.9 ± 0.71 | 0.005 | 17.75 ± 1.62 | 12.6 ± 1.9 | 0.05 | 0.001 |
| IL-6 (pg/ml) | 10.6 ± 0.41 | 4.7 ± 0.5 | 0.005 | 10.57 ± 0.31 | 9.1 ± 0.9 | 0.07 | 0.005 |
| IL-1β (pg/ml) | 14.7 ± 0.48 | 3.1 ± 0.73 | 0.001 | 14.5 ± 0.51 | 9.4 ± 0.4 | 0.03 | 0.001 |
| MCP-1 (pg/ml) | 18.7 ± 2.9 | 1.8 ± 0.9 | 0.003 | 18.1 ± 2.5 | 13.3 ± 2.1 | 0.04 | 0.001 |
| IFN-Ɣ (pg/ml) | 15.7 ± 0.61 | 8.7 ± 0.4 | 0.005 | 15.9 ± 0.51 | 12.4 ± 0.7 | 0.06 | 0.005 |
| hsCRP (mg/L) | 16.05 ± 1.01 | 2.85 ± 0.31 | 0.005 | 16.75 ± 0.8 | 7.4 ± 0.5 | 0.01 | 0.001 |
| Cardiovascular Risk Factors (CVR) | |||||||
| BP (sys/diast) | 128.3/85.3 ± 3/2 | 117/78.8 ± 2/2 | .01/.01 | 129/85 ± 3/2 | 117/79 ± 3/3 | .01/.01 | .73/.77 |
| Cholest(mg/dl) | 176 ± 14 | 152 ± 10 | 0.01 | 185 ± 13 | 170 ± 9 | 0.02 | 0.02 |
| TG (mg/dl) | 139 ± 14 | 95.8 ± 10 | 0.01 | 155 ± 13 | 159 ± 9 | 0.06 | 0.02 |
| HDL (mg/dl) | 49 ± 2 | 52 ± 2 | 0.04 | 49 ± 3 | 50 ± 2 | 0.08 | 0.05 |
| LDL (mg/dl) | 94.5 ± 4.2 | 80.4 ± 3.7 | 0.01 | 98 ± 4.4 | 95 ± 4.0 | 0.07 | 0.01 |
| FFA (mmol/L) | 0.73 ± 0.05 | 0.43 ± 0.03 | 0.001 | 0.71 ± 0.04 | 0.79 ± 0.03 | 0.04 | 0.001 |
| Oxidative Stress (ROS) | |||||||
| DCF (μM) | 4.0 ± 0.3 | 2.5 ± 0.1 | 0.01 | 4.1 ± 0.3 | 3.5 ± 0.3 | 0.04 | 0.01 |
| MDA (μM) | 2.0 ± 0.09 | 0.8 ± 0.06 | 0.01 | 2.1 ± 0.08 | 1.5 ± 0.08 | 0.04 | 0.03 |
ANALYSIS Mean ± SE were calculated.
Wilcoxon Signed Rank Test to compare baseline and 6 mo within diet group. p ≤ 0.05 was considered statistically significant.
Wilcoxon Rank Sum Test was used to compare variables between the two diet groups at 6 mo and.
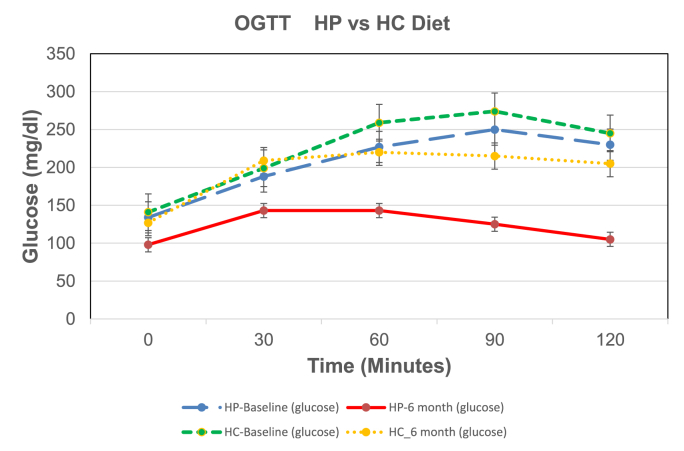
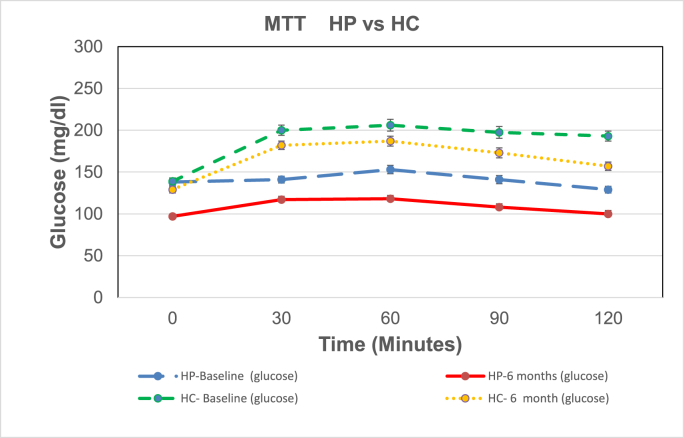
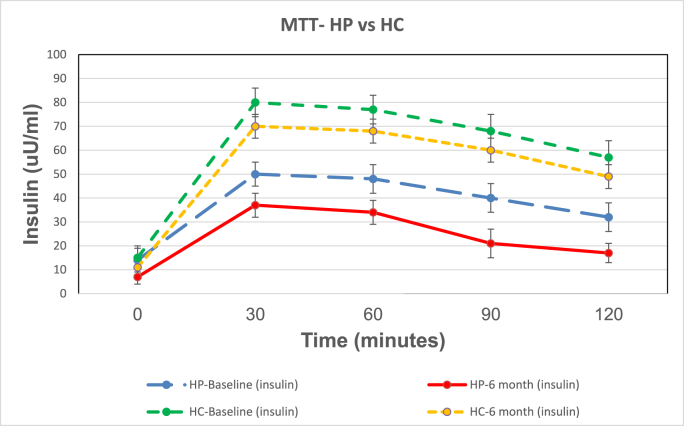
Fig. 2 A shows the mean ± SE for glucose values for the OGTT for the HP and HC diet groups. There was no significant difference of baseline (Bl) HP vs HC glucose response as shown by the figure and area under the curve (AUC). OGTT glucose response and AUCs for the HP and HC diet groups were significantly less at 6 months (mo) than at Bl. However, the OGTT glucose response AUCs for the HP diet was significantly less than the HC diet at 6 months showing greater improvement in glucose disposal with the HP diet than the HC diet. Fig. 2 B shows the mean ± SE for glucose for the MTT for the HP and HC diet groups. MTT HP vs HC glucose AUCs at Bl were significantly different demonstrating the difference in glycemic response to the HP vs HC meal of the same caloric intake (300 kcal). Glucose AUCs for the HP and HC diets at 6 months were significantly less than at Bl. However, the glucose AUCs for the HP MTT was significantly less than the glucose of the HC MTT after 6 months on the respective diets. Thus, the HP diet caused a decreased blood glucose level, greater glucose disposal and improved insulin sensitivity.
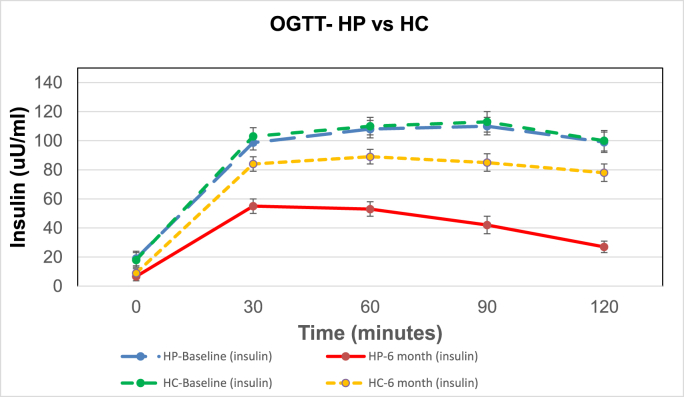
Fig. 2.
A, B, C, D. Shows the mean ± SE of glucose and insulin for the 2 h OGTTs and MTTs for the 6 HP diet subjects and the 6 HC diets subjects. The colored lines represent the following: blue line is HP diet baseline (HP_bl); red line is HP diet at 6 months (HP_6 m); green line is HC diet baseline (HC_bl); and yellow line is HC diet at 6 months (HC_6 m).
P values for the glucose AUC for the OGTTs are: HP_bl vs HP_6 m = 0.0005; HC_bl vs HC_6 m = 0.01; HP_6 m vs HC_6 m = 0.0001. P values for the glucose AUC for the MTTs are: HP_bl vs HP_6 m = 0.005; HC_bl vs HC_6 m = 0.01; HP_bl vs HC_bl = 0.001; HP_6 m vs HC_6 m = 0.001.
P values for the insulin AUC for the OGTTs are: HP bl vs HP_6 m = 0.0005; HC_bl vs HC_6 m = 0.01; HP_6 m vs HC_6 m = 0.0001.
P values for the insulin AUC for the MTTs are: HP_bl vs HP_6 m = 0.001; HC_bl vs HC_6 m = 0.01; HP_bl vs HC_bl = 0.001; HP_6 m vs HC_6 m = 0.0001. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2C shows the mean ± SE for insulin values for the OGTT for the HP and HC diet groups. There was no significant difference of the baseline (Bl) HP vs HC insulin response or AUC. Insulin response and AUCs for the HP and HC diet groups were significantly less at 6 months (mo) than at Bl. OGTT insulin AUC response for the HP diet was significantly less than the HC diet at 6 months showing a greater improvement in insulin sensitivity with the HP diet than the HC diet. Fig. 2 D shows the mean ± SE for insulin for the MTT for the HP and HC diet groups. The MTT HP vs HC insulin AUCs at Bl were significantly different demonstrating the difference in insulin response to a high protein vs high carbohydrate meal of the same caloric intake (300 kcal). Insulin AUCs for the diets at 6 months were significantly less than Bl, and insulin AUCs for the HP MTT were significantly less than HC MTT after 6 months on the respective diets, demonstrating greater insulin sensitivity and less stress on the B cells for insulin release for the same caloric intake with the HP diet.
β-hydroxybutyrate was measured as a determination of ketones on fasting blood during the study. Both groups showed no significant difference in β-hydroxybutyrate from baseline to 6 months and no significant difference between groups and demonstrated no significant ketosis induced by the diets. All chemistry profile and CBC parameters were in the normal range at baseline and 6 months. UUN increased significantly in the HP group from baseline to 6 months (8.9 ± 2.1 to 17.5 ± 2.3 gm/24 h) compared to no increase in the HC group (8.8 ± 2.2 to 9.0 ± 1.6 gm/24 h) verifying the HP group was consuming their HP diet. PTH, 25-OH Vitamin D, CrCl, microalbumin nor serum or urinary Ca levels changed significantly (data not shown) in either the HP or HC group from baseline to 6 months.
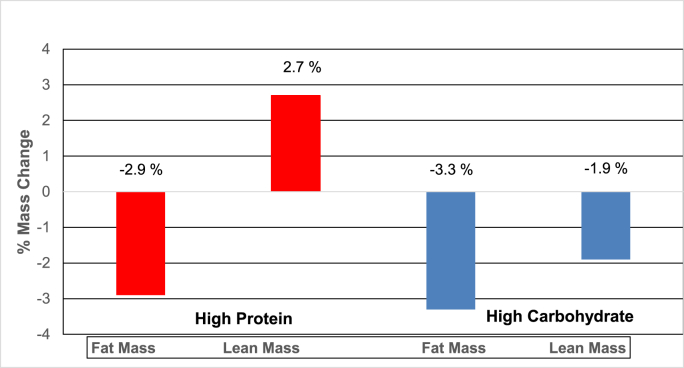
Fig. 3 shows the percent lean mass(LM) and fat mass(FM) loss in the HP and HC groups. The HP group had a significant % increase in LM while there was a significant decrease in % FM from baseline to 6 months on the HP diet. However, the HC group had a significant decrease in both the % LM and FM from baseline to 6 months on the HC diet. Showing that the HP diet group had improvement in LM while achieving weight loss.
Fig. 3.
Shows the effect of the HP and HC diets on percent changes in lean body mass (LM) and fat body mass (FM) at 6 months on the diets.
7. Conclusions
Important findings of this study which previously have not been reported are the following. 1) The HP diet resulted in 100% remission of Type 2 Diabetes to normal glucose tolerance in the subjects while the HC diet resulted in only 16% remission in those subjects. To our knowledge this is the first lifestyle intervention study where remission of T2D has been studied with 100% remission with a feeding study using meals and food obtained from local grocery stores. This study shows that remission of T2D can be achieved with dietary modification if food intake parameters are tightly controlled. 2) The HP diet group had greater improvement in insulin sensitivity, greater reduction in inflammation, oxidative stress (ROS) and cardiovascular risk factors than the HC group. 3) The % lean body mass (LM) increased while % body fat mass (FM) was decreased in the HP diet group; whereas, both % LM and FM decreased in the HC group. This preservation of % LM in the HP diet group may be an important factor in improving insulin sensitivity since muscle is a major insulin sensitive tissue for glucose uptake. 4) A high level of compliance (>90%) was obtained by both the HP and HC diet groups by giving the diet meals and menus along with survey of food consumption to the subjects at weekly visits to our CRC.
Since the American Diabetes Association recommends that subjects with T2D exercise 30 min per day, all subjects were asked to walk for 30 min per day and this was monitored by Fitbits given to the subjects. There was no significant difference in amount of exercise between the HP and HC diet groups; therefore, not affecting the results. Although the OGTT glucose levels were similar at baseline, the HC group sustained significantly higher glucose levels remaining in the T2D range compared to the HP diet group whose levels decreased to normal glucose levels after 6 months on the diet. The HP MTT had significantly lower glucose levels at baseline and at 6 months than the HC MTT. The increased improvement in insulin sensitivity and Beta cell function with the HP group compared to the HC group likely equates to decreased β cells stress in the HP group. Our study demonstrates that higher sustained glucose elevation with ingestion of glucose or higher glycemic foods as in the HC diet correlates with increased inflammation and oxidative stress in the HC group compared to the HP group. Antioxidant enzymes induced by repeated intake of excess energy in the form of high carbohydrate or high fat diets are not sufficient to block oxidative stress and inflammation has been shown [75]. Therefore, the fact that our HP diet had a significantly greater reduction in inflammation, ROS, and cardiovascular risk factor markers than the HC diet in Type 2 Diabetes subjects is of great health significance.
Our HP and HC diets contained more than the FDA recommended amount of Ca/day [67] and showed no bone loss or loss of Ca in the urine [76], in contrast to a study that reported a HP diet can cause negative calcium (Ca) balance, increased Ca loss in the urine and adversely affect the bone [25]. Our HP diet (30% protein) is at the upper limit of suggested protein consumption range (10–30%) which may not be at a sufficient percentage to cause a negative calcium (Ca) balance.
The majority of the subjects in our study were African American (AA) subjects and neither baseline HOMA-IR or ISI (Matsuda Index) were significantly different between the HP and HC groups; although possible limitation in assessment of insulin sensitivity by HOMA-IR with AA has been suggested [77]. However, both methods of assessment showed greater improvement of insulin sensitivity with the HP diet than the HC diet at 6 months.
Compliance of diet is an important factor in macronutrient composition diet studies. Strengths of this feeding study are that it was a randomized control trial where all food and daily diet plans were provided to each subject in both the HP and HC diet groups at their weekly visit to the CRC along with daily menu consumption survey each week for 6 months. This monitoring resulted in greater than 90% compliance of diet adherence. A patient's recall food questionnaire of food they ate days to weeks before is used in most studies which is generally inaccurate. Unlike weight loss counseling studies which rely solely on subject's self-determined food selections (DPP, Look Ahead) [6,78] and the liquid diet reported by Lean [79], we provided each subject with an individualized menu based on weight loss needs that map out what they are to eat each day for 6 months using foods obtained from local grocery stores. Our HP diet was 30% protein which is not excessive yet preserves muscle mass while not causing any liver or kidney problems; whereas, the high carbohydrate diet was 15% protein and results in loss of % lean body mass.
Our study demonstrates that strict adherence of dietary intervention is possible and can produce meaningful and reliable results and remission of Type 2 Diabetes. Subjects that chose to stay on the HP diet plans for an additional 6 months purchasing their own food and consultations with the investigators when needed maintained their weight loss and normal glucose levels at the 6 months follow up visit.
The meal plans are adjustable to the subject's food likes and dislike with a wide variety of choices with different meal plans for each day of the week consisting of foods which are available at local grocery stores at a daily cost of around thirteen dollars. Initial instructions and weekly meal plans could be provided to the subject along with phone and email consultation when needed. By this method the HP diet plan would allow for a diet care plan to be instituted by primary care physicians and offer an economical means of a nutritional weight loss diet and remission of Type 2 Diabetes in both women and men.
Type 2 diabetes is considered to be an inflammatory disease [80] and as we and others have shown these subjects have inflammatory cytokines as do subjects with ARDS, COVID-19 [81,82] and can exasperate the inflammation response. Decreasing the inflammatory cytokines with remission of Type 2 Diabetes can help prevent other inflammatory diseases from becoming a cytokine storm of inflammation and decrease cardiovascular risk factors.
The HP diet although isocaloric with the HC diet has demonstrated significant metabolic improvements compared to the HC diet. This study shows that changing the macronutrients can improve cardiovascular risk factors, inflammation, per cent lean and fat mass weight loss, and attenuate insulin action. This study is unique in that we used a non-pharmaceutical means (HP diet) for remission of T2D and weight loss, as we have used in our previous studies of prediabetes remission, to achieve remission of T2D, weight loss and decreased inflammation, oxidative stress and cardiovascular risk factors.
Funding statement
The study was funded by the American Diabetes Association (7-12-CT-41) and the A.D. Baskin Research Fund (PI F. Stentz)
Contribution statement
Author contributions: F·B.S. wrote the manuscript, researched data and contributed to the conception, design, coordinated recruitment and following subjects on the study, C.S. conducted history and physical examinations of subjects, reviewed data and manuscript. D.L., S.T., and J.C. performed some of the laboratory assays, reviewed and edited manuscript and organized patient data. Nutritionists at the UTHSC CRC provided information on diets and provided daily food and menus to participants, Dr. Jim Wan was the biostatistician in charge of statistical analysis. F.B.S. is the guarantor of this work and, as such, had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The manuscript has been read and approved by all the authors.
Disclosure statement
The authors have nothing to disclose.
Data sharing statement
No additional data are available.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgements
The authors thank A. Ammons at UTHSC for assays of hormones and cytokines, all of the nursing and recruiting staff of the Clinical Research Center unit, all of whom are affiliated with the UTHSC, for their efforts and for assisting with some of the OGTT and MTTs.. The authors also thank all the study volunteers for their participation in the study. The Medical Student Research Fellowship Program from the National Institutes of Health/National Institute of Diabetes and Digestive Diseases (C5T35DK007405-28) (S. Dagogo-Jack, PI) at the University of Tennessee Health Science Center funded participation of second year medical students Damon Lawson and Sidney Tucker of this research study.
References
- 1.Center for Disease Control C. National diabetes Statistics Report. 2022. www.cdc.gov/diabetes/data/statistics/2022
- 2.Hu F.B., Manson J.E., Stampfer M.J., Colditz G., Liu S., Solomon C.G., Willett W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 3.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control C. National obesity Statistics Report. 2020. www.cdc.gov/obesity/adult/index 2020.
- 5.Manson J.E., Willett W.C., Stampfer M.J., Colditz G.A., Hunter D.J., Hankinson S.E., Hennekens C.H., Speizer F.E. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program The diabetes prevention program (DPP) research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research G., Knowler W.C., Fowler S.E., Hamman R.F., Christophi C.A., Hoffman H.J., Brenneman A.T., Brown-Friday J.O., Goldberg R., Venditti E., Nathan D.M. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo R.A., Tripathy D., Schwenke D.C., Banerji M., Bray G.A., Buchanan T.A., Clement S.C., Henry R.R., Hodis H.N., Kitabchi A.E., Mack W.J., Mudaliar S., Ratner R.E., Williams K., Stentz F.B., Musi N., Reaven P.D. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A., De Nigris V., Prattichizzo F. Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes Metabol. 2020;22(10):1951–1952. doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Li H., Zhang J., Cao Y., Zhao X., Yu N., Gao Y., Ma J., Zhang H., Zhang J., Guo X., Liu X. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metabol. 2020;22(8):1443–1454. doi: 10.1111/dom.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddaloni E., Buzzetti R. Diabetes Metab Res Rev; 2020. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics; p. e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perreault L., Pan Q., Mather K.J., Watson K.E., Hamman R.F., Kahn S.E., Diabetes Prevention Program Research G. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B., Liu P.A., Jiang X.G., Jiang Y.Y., Wang J.P., Zheng H., Zhang H., Bennett P.H., Howard B.V. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 14.Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., Salminen V., Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 15.Defronzo R.A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyenwe E.A., Jerkins T.W., Umpierrez G.E., Kitabchi A.E. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60(1):1–23. doi: 10.1016/j.metabol.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo R.A., Banerji M.A., Bray G.A., Buchanan T.A., Clement S., Henry R.R., Kitabchi A.E., Mudaliar S., Musi N., Ratner R., Reaven P., Schwenke D.C., Stentz F.D., Tripathy D. Determinants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW) study. Diabetologia. 2010;53(3):435–445. doi: 10.1007/s00125-009-1614-2. [DOI] [PubMed] [Google Scholar]
- 18.Franz M.J., Powers M.A., Leontos C., Holzmeister L.A., Kulkarni K., Monk A., Wedel N., Gradwell E. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110(12):1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Larsen R.N., Mann N.J., Maclean E., Shaw J.E. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia. 2011;54(4):731–740. doi: 10.1007/s00125-010-2027-y. [DOI] [PubMed] [Google Scholar]
- 20.Alford B.B., Blankenship A.C., Hagen R.D. The effects of variations in carbohydrate, protein, and fat content of the diet upon weight loss, blood values, and nutrient intake of adult obese women. J Am Diet Assoc. 1990;90(4):534–540. [PubMed] [Google Scholar]
- 21.McManus K., Antinoro L., Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord. 2001;25(10):1503–1511. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- 22.Larsen T.M., Dalskov S.M., van Baak M., Jebb S.A., Papadaki A., Pfeiffer A.F., Martinez J.A., Handjieva-Darlenska T., Kunesova M., Pihlsgard M., Stender S., Holst C., Saris W.H., Astrup A. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann A.J., Nordmann A., Briel M., Keller U., Yancy W.S., Jr., Brehm B.J., Bucher H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 24.Boden G., Sargrad K., Homko C., Mozzoli M., Stein T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142(6):403–411. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Eisenstein J., Roberts S.B., Dallal G., Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60(7 Pt 1):189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 26.Bray G.A., Smith S.R., de Jonge L. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association A. Standard of medical care in diabetes. Diabetes Care. 2020;43(Suppl 1):S1–S7. [Google Scholar]
- 28.Stentz F.B., Brewer A., Wan J., Garber C., Daniels B., Sands C., Kitabchi A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care. 2016;4(1):e000258. doi: 10.1136/bmjdrc-2016-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitabchi A.E., Stentz F.B., McDaniel K.A., Wan J.Y., Nyenwe E., Sands C.W. Effects of high-protein versus high-carbohydrate diets on markers of beta-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes: a randomized controlled trial. Diabetes Care. 2013;36(7):1919–1925. doi: 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stentz F., Kimeish O., Kitabchi A. Effect of high protein vs high carbohydrate diets on incretins, satiety and cardiovascular factors. Diabetes. 2014;62(Suppl 1):1825. [Google Scholar]
- 31.Jenkins D.J., Wolever T.M., Taylor R.H., Barker H., Fielden H., Baldwin J.M., Bowling A.C., Newman H.C., Jenkins A.L., Goff D.V. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig D.S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 33.Robinson S.M., Jaccard C., Persaud C., Jackson A.A., Jequier E., Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr. 1990;52(1):72–80. doi: 10.1093/ajcn/52.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Samaha F.F., Iqbal N., Seshadri P., Chicano K.L., Daily D.A., McGrory J., Williams T., Williams M., Gracely E.J., Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 35.Caminhotto R.O., Fonseca F.L., Castro N.C., Arantes J.P., Sertie R.A. Arch Endocrinol Metab; 2015. Atkins diet program rapidly decreases atherogenic index of plasma in trained adapted overweight men. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins D.J., Kendall C.W., Vidgen E., Augustin L.S., van Erk M., Geelen A., Parker T., Faulkner D., Vuksan V., Josse R.G., Leiter L.A., Connelly P.W. High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr. 2001;74(1):57–63. doi: 10.1093/ajcn/74.1.57. [DOI] [PubMed] [Google Scholar]
- 37.Larosa J.C., Fry A.G., Muesing R., Rosing D.R. Effects of high-protein, low-carbohydrate dieting on plasma lipoproteins and body weight. J Am Diet Assoc. 1980;77(3):264–270. [PubMed] [Google Scholar]
- 38.Nuttall F.Q., Mooradian A.D., Gannon M.C., Billington C., Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7(5):465–470. doi: 10.2337/diacare.7.5.465. [DOI] [PubMed] [Google Scholar]
- 39.Krezowski P.A., Nuttall F.Q., Gannon M.C., Bartosh N.H. The effect of protein ingestion on the metabolic response to oral glucose in normal individuals. Am J Clin Nutr. 1986;44(6):847–856. doi: 10.1093/ajcn/44.6.847. [DOI] [PubMed] [Google Scholar]
- 40.Stentz F.B., Mikhael A., Kineish O., Christman J., Sands C. High protein diet leads to prediabetes remission and positive changes in incretins and cardiovascular risk factors. Nutr Metabol Cardiovasc Dis. 2021;31(4):1227–1237. doi: 10.1016/j.numecd.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Stentz F.B., Mikhael A., Kineish O., Christman J.V., Sands C. Incretins and cardiovascular effects of weight loss and remission of prediabetes. J Diabet Clin Studies (JDCS) 2020;3(1):1–8. [Google Scholar]
- 42.Eng C., Kramer C.K., Zinman B., Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228–2234. doi: 10.1016/S0140-6736(14)61335-0. [DOI] [PubMed] [Google Scholar]
- 43.Ahren B., Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2004;36(11–12):867–876. doi: 10.1055/s-2004-826178. [DOI] [PubMed] [Google Scholar]
- 44.Davidson J.A. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med. 2009;76(Suppl 5):S28–S38. doi: 10.3949/ccjm.76.s5.05. [DOI] [PubMed] [Google Scholar]
- 45.Young L.A., Buse J.B. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384(9961):2180–2181. doi: 10.1016/S0140-6736(14)61409-4. [DOI] [PubMed] [Google Scholar]
- 46.Kim W., Egan J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle P.J., Freeman J.S. Application of incretin mimetics and dipeptidyl peptidase IV inhibitors in managing type 2 diabetes mellitus. J Am Osteopath Assoc. 2007;107(Suppl):S10–S16. [PubMed] [Google Scholar]
- 48.Stentz F.B., Ammons A., Sands C. Incretin and cardiovascular effects of weight loss and remission of prediabetes. Diabetes. 2018;67(Suppl 1):544. [Google Scholar]
- 49.Stentz F.B., Eastman A., Christman J.V. Hyperglycemia and hyperlipidemia induced inflammation and oxidative stress in human T lymphocytes and salutary effects of ω- 3 fatty acid. SunKrist J Diabet Clin Care. 2020;1:1–9. [Google Scholar]
- 50.Stentz F.B., Kitabchi A.E. Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun. 2005;335(2):491–495. doi: 10.1016/j.bbrc.2005.07.109. [DOI] [PubMed] [Google Scholar]
- 51.Stentz F.B., Kitabchi A.E. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem Biophys Res Commun. 2006;346(3):721–726. doi: 10.1016/j.bbrc.2006.05.159. [DOI] [PubMed] [Google Scholar]
- 52.Mohanty P., Hamouda W., Garg R., Aljada A., Ghanim H., Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85(8):2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 53.Stentz F.B., Umpierrez G.E., Cuervo R., Kitabchi A.E. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 54.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Meduri G.U., Kohler G., Headley S., Tolley E., Stentz F., Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 56.Meduri G.U., Headley S., Tolley E., Shelby M., Stentz F., Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. 1995;108(5):1315–1325. doi: 10.1378/chest.108.5.1315. [DOI] [PubMed] [Google Scholar]
- 57.Meduri G.U., Tolley E.A., Chrousos G.P., Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165(7):983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 58.Meduri G.U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R., Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 59.Darif D., Hammi I., Kihel A., El Idrissi Saik I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piatti P.M., Monti F., Fermo I., Baruffaldi L., Nasser R., Santambrogio G., Librenti M.C., Galli-Kienle M., Pontiroli A.E., Pozza G. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism. 1994;43(12):1481–1487. doi: 10.1016/0026-0495(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 61.Layman D.K., Boileau R.A., Erickson D.J., Painter J.E., Shiue H., Sather C., Christou D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133(2):411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 62.Gleason J.A., Bourdet K.L., Koehn K., Holay S.Y., Schaefer E.J. Cardiovascular risk reduction and dietary compliance with a home-delivered diet and lifestyle modification program. J Am Diet Assoc. 2002;102(10):1445–1451. doi: 10.1016/s0002-8223(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 63.Howard B.V., Manson J.E., Stefanick M.L., Beresford S.A., Frank G., Jones B., Rodabough R.J., Snetselaar L., Thomson C., Tinker L., Vitolins M., Prentice R. Low-fat dietary pattern and weight change over 7 years: the women's health initiative dietary modification trial. JAMA. 2006;295(1):39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 64.Gardner C.D., Kiazand A., Alhassan S., Kim S., Stafford R.S., Balise R.R., Kraemer H.C., King A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 65.Tripathy D., Mohanty P., Dhindsa S., Syed T., Ghanim H., Aljada A., Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 66.Haffner S.M. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol. 2003;92(4A):18J–26J. doi: 10.1016/s0002-9149(03)00612-x. [DOI] [PubMed] [Google Scholar]
- 67.NIH Consensus conference. Optimal calcium intake. NIH consensus development panel on optimal calcium intake. JAMA. 1994;272(24):1942–1948. [PubMed] [Google Scholar]
- 68.Institute of Medicine . vol. 1. National Academy Press; Washington D.C.: 2005. (Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids). [DOI] [PubMed] [Google Scholar]
- 69.Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B., Eckel R.H., Goldberg A.C., Gordon D., Levy D., Lloyd-Jones D.M., McBride P., Schwartz J.S., Shero S.T., Smith S.C., Jr., Watson K., Wilson P.W., Eddleman K.M., Jarrett N.M., LaBresh K., Nevo L., Wnek J., Anderson J.L., Halperin J.L., Albert N.M., Bozkurt B., Brindis R.G., Curtis L.H., DeMets D., Hochman J.S., Kovacs R.J., Ohman E.M., Pressler S.J., Sellke F.W., Shen W.K., Smith S.C., Jr., Tomaselli G.F., American G. College of cardiology/American heart association task force on practice. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 70.Larkin F.A., Metzner H.L., Guire K.E. Comparison of three consecutive-day and three random-day records of dietary intake. J Am Diet Assoc. 1991;91(12):1538–1542. [PubMed] [Google Scholar]
- 71.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 72.Stentz F.B. In: Hyperglycemia- and hyperlipidemia- induced inflammation and oxidative stress through human T-Lymphocytes and human aortic endothelial cells (HAEC) Sugars R. Rovan., editor. IntechOpen, Limited; London, England: 2021. pp. 1–16. [Google Scholar]
- 73.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 74.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 75.Lim S., Won H., Kim Y., Jang M., Jyothi K.R., Dandona P., Ha J., Kim S.S. Antioxidant enzymes induced by repeated intake of excess energy in the form of high-fat, high-carbohydrate meals are not sufficient to block oxidative stress in healthy lean individuals. Br J Nutr. 2011;106(10):1544–1551. doi: 10.1017/S0007114511002091. [DOI] [PubMed] [Google Scholar]
- 76.Stentz F., Garber C., Kitabchi A., Sands C. Efficacy of High Protein vs. High Carbohydrate Diet on Remission of Impaired Glucose Tolerance (IGT) to Normal Glucose Tolerance (NGT) Diabetes. 2015;64(suppl 1):512. [Google Scholar]
- 77.Pisprasert V., Ingram K.H., Lopez-Davila M.F., Munoz A.J., Garvey W.T. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845–853. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gregg E.W., Chen H., Wagenknecht L.E., Clark J.M., Delahanty L.M., Bantle J., Pownall H.J., Johnson K.C., Safford M.M., Kitabchi A.E., Pi-Sunyer F.X., Wing R.R., Bertoni A.G., Look A.R.G. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., Rodrigues A.M., Rehackova L., Adamson A.J., Sniehotta F.F., Mathers J.C., Ross H.M., McIlvenna Y., Stefanetti R., Trenell M., Welsh P., Kean S., Ford I., McConnachie A., Sattar N., Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 80.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 81.Wilson J.G., Simpson L.J., Ferreira A.M., Rustagi A., Roque J., Asuni A., Ranganath T., Grant P.M., Subramanian A., Rosenberg-Hasson Y., Maecker H.T., Holmes S.P., Levitt J.E., Blish C.A., Rogers A.J. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sciences N.I.o.E.H. Inflammation. NIH. 2022;1:1–20. https://www.niehs.nih.gov [Google Scholar]