Abstract
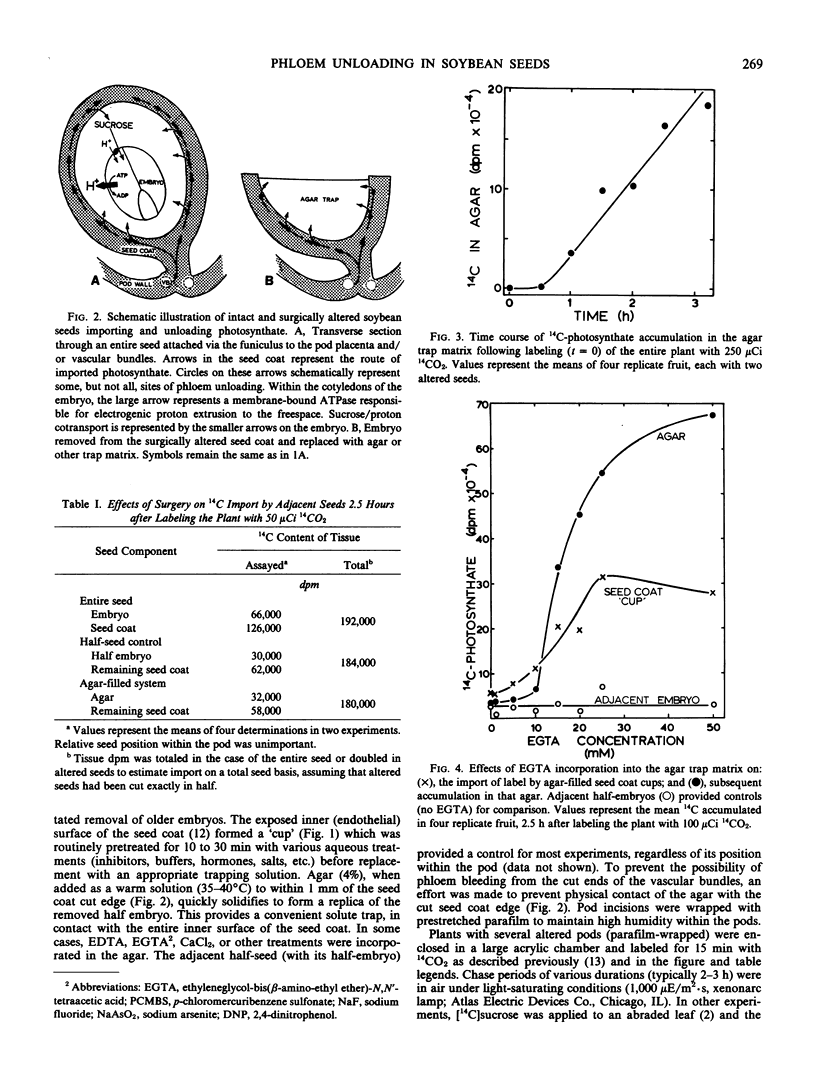
A technique has been developed which permits mechanistic studies of phloem unloading in developing seeds of soybean (Glycine max cv Clark) and other legumes. An opening is cut in the pod wall and the embryo surgically removed from the seedcoat without diminishing the capacity of that tissue for assimilate import, phloem unloading, or efflux. The sites of phloem unloading were accessible via the seedcoat apoplast and were challenged with inhibitors, solutes, buffers, etc., to characterize the unloading process.
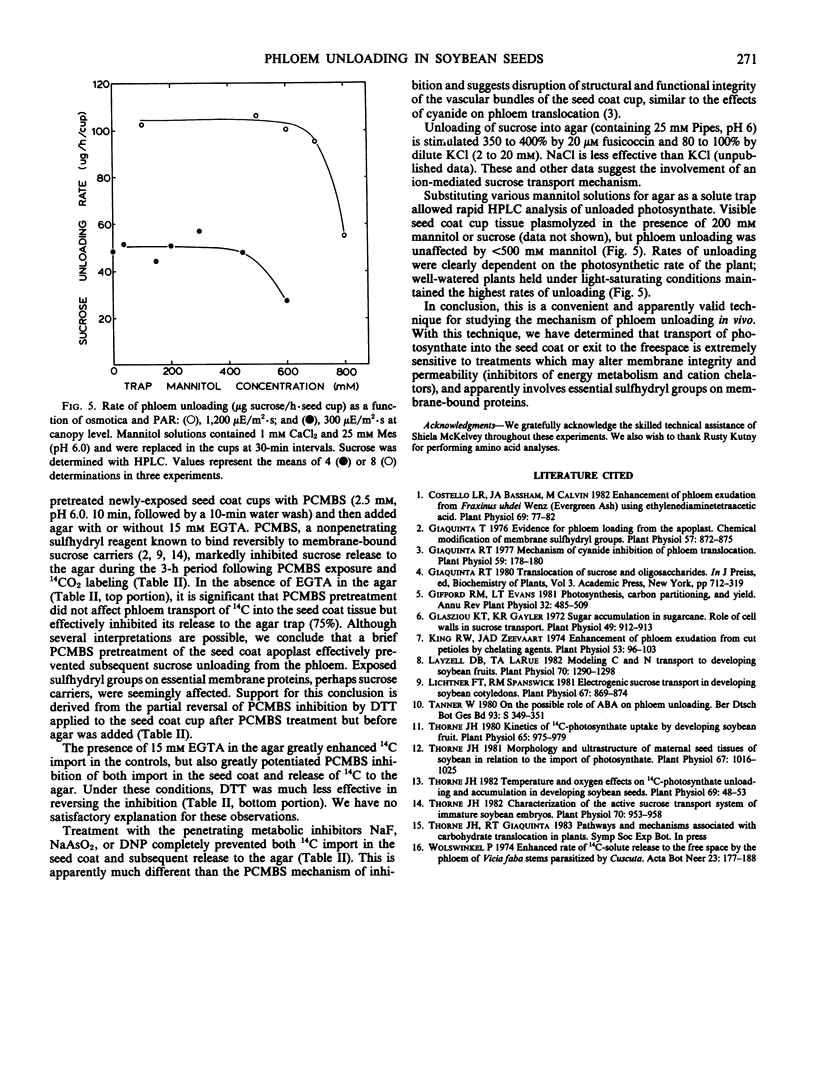
Unloading is stimulated by divalent metal chelators and diethylstilbestrol, and inhibited by metabolic uncouplers and sulfhydryl group modifiers. Solutes released from the seed coat had a carbon/nitrogen ratio of 31 milligrams carbon per milligram nitrogen; sucrose represented 90% of the carbon present and various nitrogenous solutes contributed the remaining 10%. Unloading could be maintained for up 8 hours at rates of 0.5 to 1.0 micromoles per hour, providing a valid, convenient in vivo technique for studies of phloem unloading and seed growth mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costello L. R., Bassham J. A., Calvin M. Enhancement of Phloem Exudation from Fraxinus uhdei Wenz. (Evergreen Ash) using Ethylenediaminetetraacetic Acid. Plant Physiol. 1982 Jan;69(1):77–82. doi: 10.1104/pp.69.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Mechanism of cyanide inhibition of Phloem translocation. Plant Physiol. 1977 Feb;59(2):178–180. doi: 10.1104/pp.59.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou K. T., Gayler K. R. Sugar accumulation in sugarcane: role of cell walls in sucrose transport. Plant Physiol. 1972 Jun;49(6):912–913. doi: 10.1104/pp.49.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Characterization of the active sucrose transport system of immature soybean embryos. Plant Physiol. 1982 Oct;70(4):953–958. doi: 10.1104/pp.70.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Kinetics of C-photosynthate uptake by developing soybean fruit. Plant Physiol. 1980 May;65(5):975–979. doi: 10.1104/pp.65.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Morphology and ultrastructure of maternal seed tissues of soybean in relation to the import of photosynthate. Plant Physiol. 1981 May;67(5):1016–1025. doi: 10.1104/pp.67.5.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Temperature and oxygen effects on C-photosynthate unloading and accumulation in developing soybean seeds. Plant Physiol. 1982 Jan;69(1):48–53. doi: 10.1104/pp.69.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]