Abstract
Photosynthetically active chloroplasts retaining high rates of fatty acid synthesis from [1-14C]acetate were purified from leaves of both 16:3 (Solanum nodiflorum, Chenopodium album) and 18:3 plants (Amaranthus lividus, Pisum sativum). A comparison of lipids into which newly synthesized fatty acids were incorporated revealed that, in 18:3 chloroplasts, enzymic activities catalyzing the conversion of phosphatidate to diacylglycerol and of diacylglycerol to monogalactosyl diacylglycerol (MGD) were significantly less active than in 16:3 chloroplasts. In contrast, labeling rates of MGD from UDP-[14C]gal were similar for both types of chloroplasts.
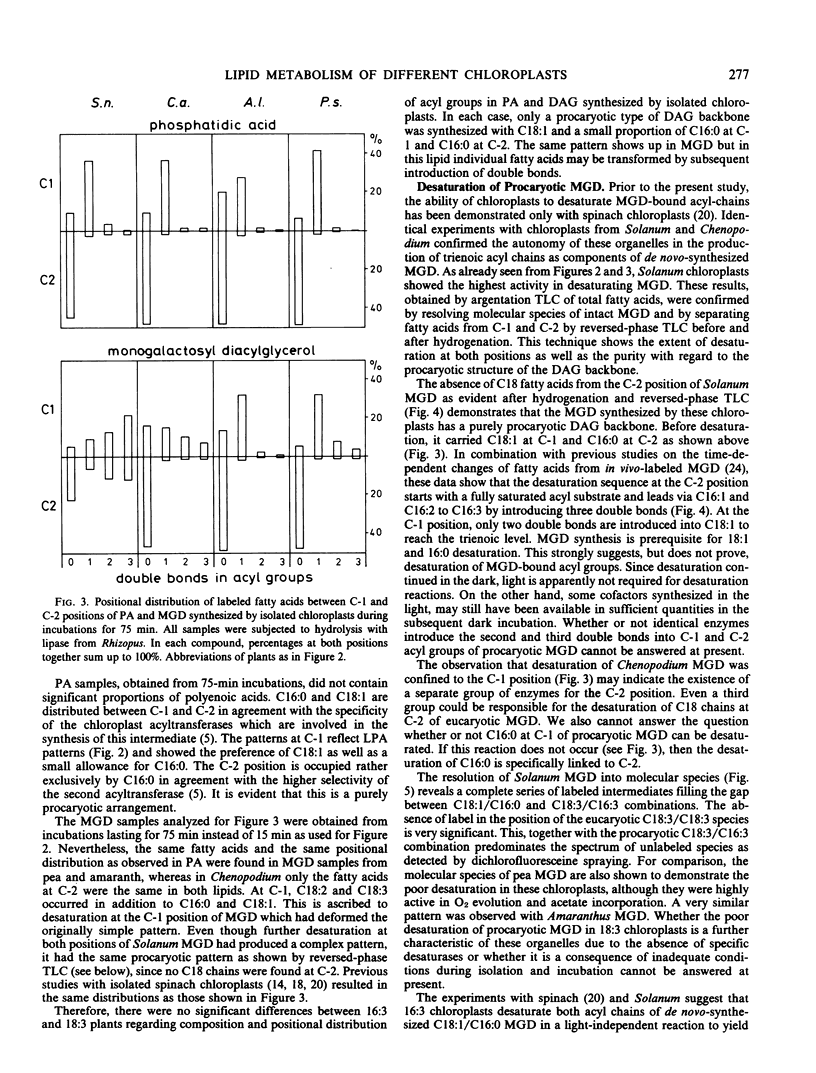
The composition and positional distribution of labeled fatty acids within the glycerides synthesized by isolated 16:3 and 18:3 chloroplasts were similar and in each case only a C18/C16 diacylglycerol backbone was synthesized. In nodiflorum chloroplasts, C18:1/C16:0 MGD assembled de novo was completely desaturated to the C18:3/C16:3 stage.
Whereas newly synthesized C18/C18 MGD could not be detected in any of these chloroplasts if incubated with [14C]acetate after isolation, chloroplasts isolated from acetate-labeled leaves contained MGD with labeled C18 fatty acids at both sn-1 and sn-2 positions. Taken together, these results provide further evidence on an organellar level for the operation of pro- and eucaryotic pathways in the biosynthesis of MGD in different groups of plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A. A simple and convenient procedure for the hydrogenation of lipids on the micro- and nanomole scale. J Lipid Res. 1972 Jan;13(1):146–148. [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Hajra A. K. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974 Aug;9(8):502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Knauf M., Stumpf P. K. Subcellular localization of acetyl-CoA synthetase in leaf protoplasts of Spinacia oleracea. Arch Biochem Biophys. 1981 Jul;209(2):441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- Mudd J. B., Dezacks R. Synthesis of phosphatidylglycerol by chloroplasts from leaves of Spinacia oleracea L. (spinach). Arch Biochem Biophys. 1981 Jul;209(2):584–591. doi: 10.1016/0003-9861(81)90316-7. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Mudd J. B., McManus T. T., Slack C. R. Linoleate and alpha-linolenate synthesis by isolated spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Dec 15;184(3):571–574. doi: 10.1042/bj1840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Turnover of the glycerolipids of pumpkin leaves. The importence of phosphatidylcholine. Biochem J. 1970 Mar;117(1):1–8. doi: 10.1042/bj1170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Murata N., Miura Y., Ueta N. Effect of growth temperature on lipid and fatty acid compositions in the blue-green algae, Anabaena variabilis and Anacystis nidulans. Biochim Biophys Acta. 1979 Jan 29;572(1):19–28. [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. The function of acyl thioesterases in the metabolism of acyl-coenzymes A and acyl-acyl carrier proteins. Arch Biochem Biophys. 1976 Jan;172(1):110–116. doi: 10.1016/0003-9861(76)90054-0. [DOI] [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Joyard J., Douce R. Labelling in vivo and in vitro of molecular species of lipids from chloroplast envelopes and thylakoids. Eur J Biochem. 1980;108(1):177–185. doi: 10.1111/j.1432-1033.1980.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]