Abstract
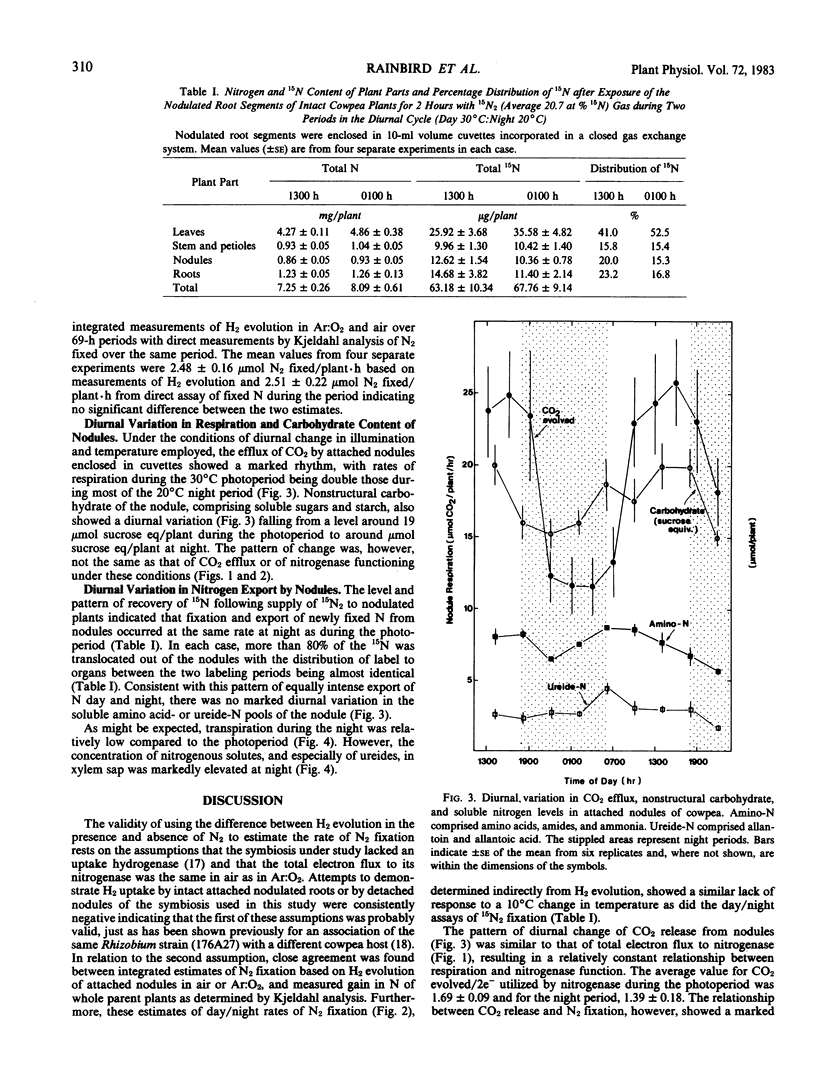
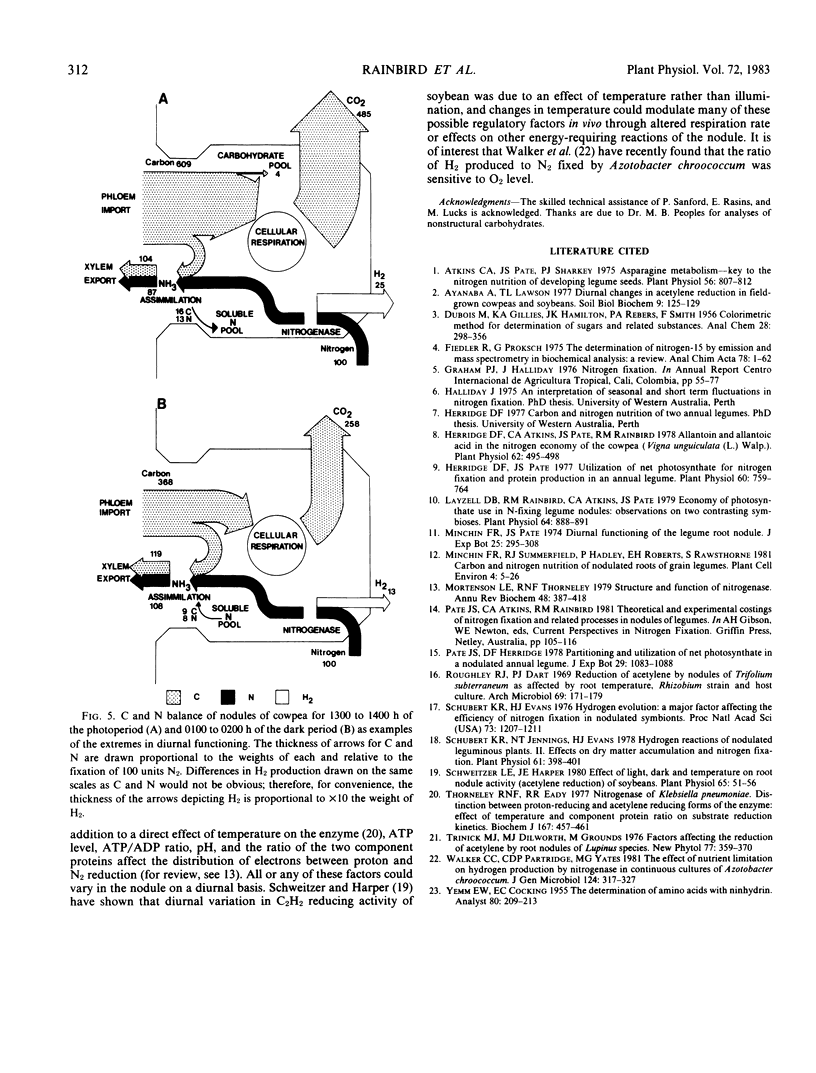
Nitrogenase (EC 1.7.99.2) activity of nodules of cowpea (Vigna unguiculata [L.] Walp), maintained under conditions of a 12-hour day at 30°C and 800 to 1,000 microeinsteins per square meter per second (photosynthetically active radiation) and a 12-hour night at 20°C, showed a marked diurnal variation with the total electron flux through the enzyme at night being 60% of that in the photoperiod. This diurnal pattern was, however, due to changes in hydrogen evolution. The rate of nitrogen fixation, measured by short-term 15N2 assimilation or estimated from the difference in hydrogen evolution in air or Ar:O2 (80:20; v/v), showed no diurnal variation. Carbon dioxide released from nodules showed a diurnal variation synchronized with that of nitrogenase functioning and, as a consequence, the apparent `respiratory cost' of nitrogen fixation in the photoperiod was almost double that at night (9.74 ± 0.38 versus 5.70 ± 0.90 moles CO2 evolved per mole N2 fixed). Separate carbon and nitrogen balances constructed for nodules during the photoperiod and dark period showed that, at night, nodule functioning required up to 40% less carbohydrate to achieve the same level of nitrogen fixation as during the photoperiod (2.4 versus 1.4 moles hexose per mole N2 fixed).
Stored reserves of nonstructural carbohydrate of the nodule only partly satisfied the requirement for carbon at night, and fixation was dependent on continued import of translocated assimilates at all times. Measurements of the soluble nitrogen pools of the nodule together with 15N studies indicated that, both during the day and night, nitrogenous products of fixation were effectively translocated to all organs of the host plant despite low rates of transpiration at night. Reduced fluxes of water through the plant at night were apparently counteracted by increased concentration of nitrogen, especially as ureides, in the xylem stream.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Pate J. S. Utilization of net photosynthate for nitrogen fixation and protein production in an annual legume. Plant Physiol. 1977 Nov;60(5):759–764. doi: 10.1104/pp.60.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer L. E., Harper J. E. Effect of light, dark, and temperature on root nodule activity (acetylene reduction) of soybeans. Plant Physiol. 1980 Jan;65(1):51–56. doi: 10.1104/pp.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Eady R. R. Nitrogenase of Klebsiella pneumoniae. Distinction between proton-reducing and acetylene-reducing forms of the enzyme: effect of temperature and component protein ratio on substrate-reduction kinetics. Biochem J. 1977 Nov 1;167(2):457–461. doi: 10.1042/bj1670457. [DOI] [PMC free article] [PubMed] [Google Scholar]


