Abstract
Light induced an alkalinization and stimulated a subsequent acidification of the medium surrounding oat (Avena sativa L. cv Garry) leaf protoplasts. Blue light was less effective than would be predicted from photosynthetic action spectra. Nonetheless, 3-(3,4-dichlorophenyl)-1,1-dimethylurea prevented alkalinization and reduced acidification to the dark rate for protoplast suspensions exposed to all light regimes tested.
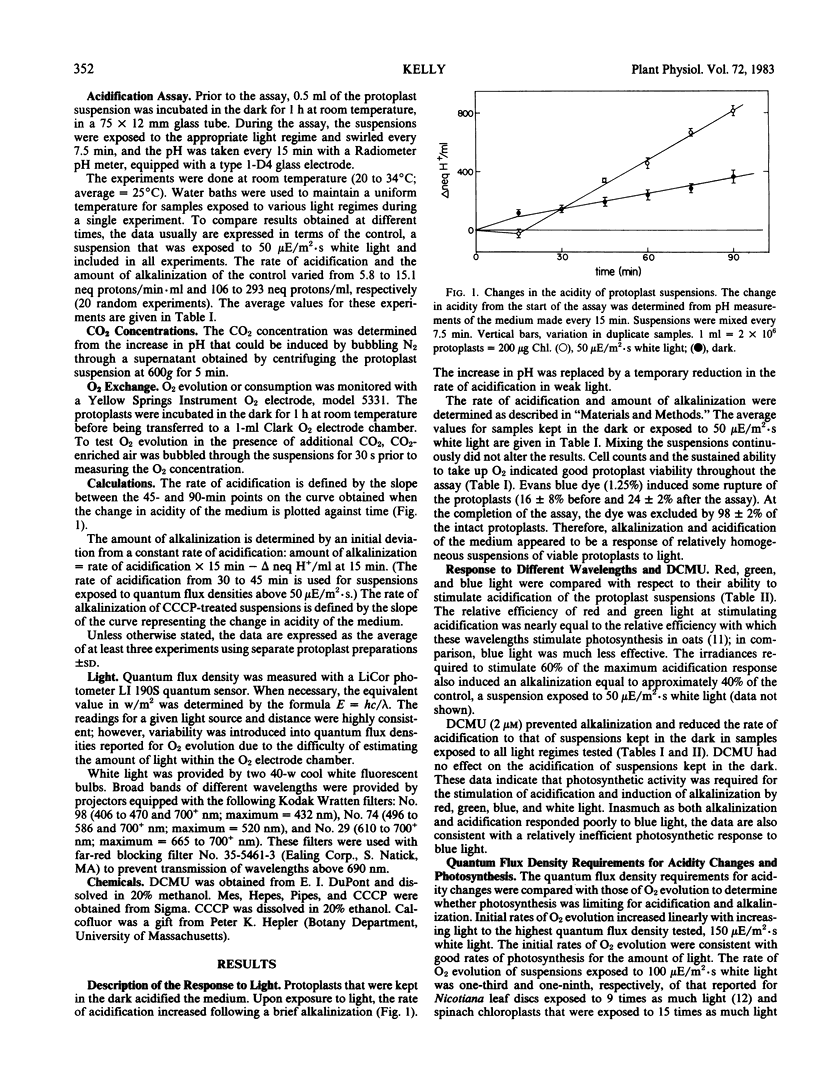
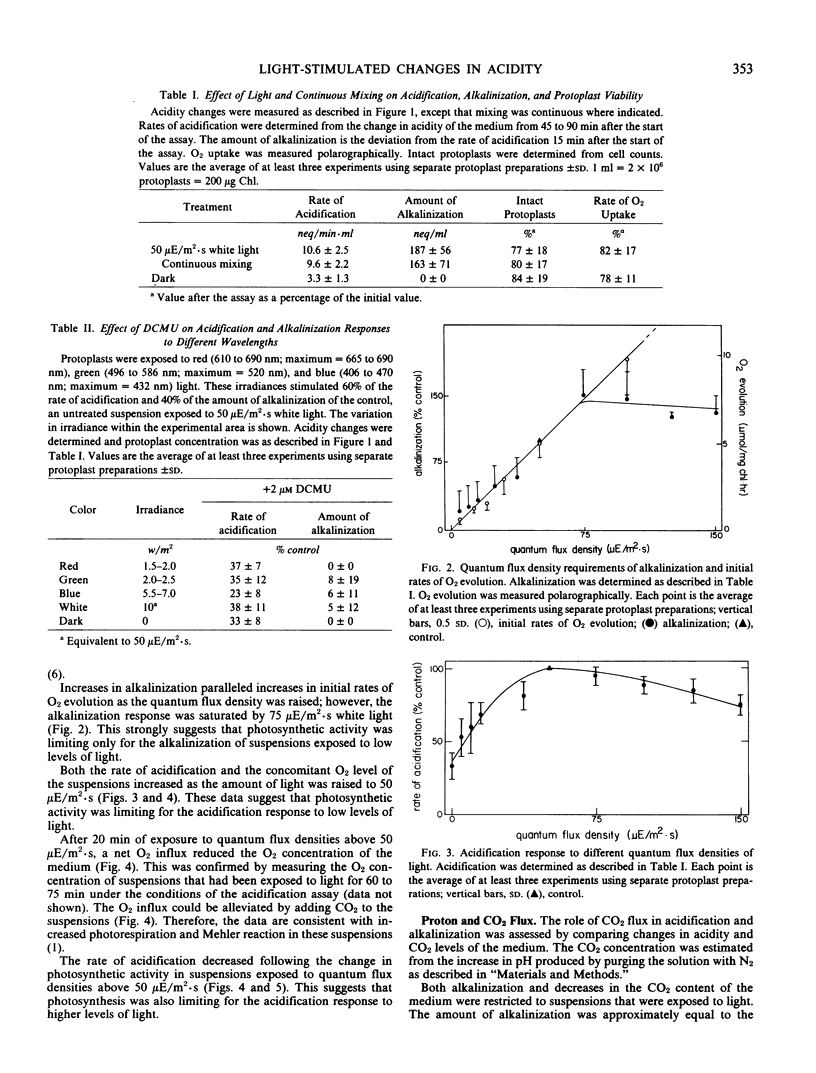
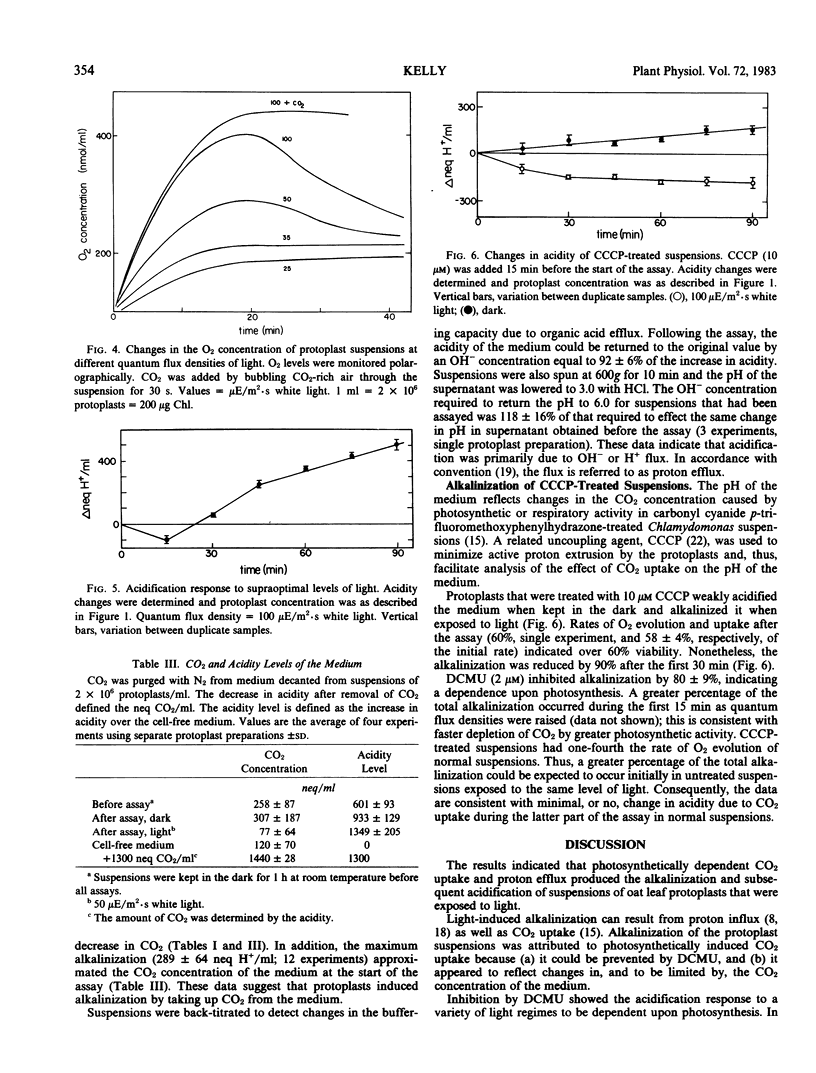
Alkalinization increased in parallel with initial rates of O2 evolution as the quantum flux density of white light was raised to 75 microeinsteins per square meter per second. Alkalinization was accompanied by a decrease in the CO2 content of the medium; therefore, it was attributed to photosynthetically induced CO2 uptake. The effect of CO2 depletion on the acidity of the medium appeared to be mainly restricted to the first 15 minutes of exposure to light. Consequently, subsequent pH changes primarily reflected a constant net proton efflux. Acidification occurred in the dark, but rates of acidification increased in response to increased light approximately in parallel with changes in a concomitant net O2 efflux. The results indicated that protoplasts could acidify the medium in response to nonphotosynthetic activity, but that photosynthesis mediated light stimulation of acidification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens P. W., Marsho T. V., Radmer R. J. Photosynthetic o(2) exchange kinetics in isolated soybean cells. Plant Physiol. 1982 Jul;70(1):179–185. doi: 10.1104/pp.70.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee C., Kendrick R. E. Ion Fluxes and Phytochrome Protons in Mung Bean Hypocotyl Segments: II. Fluxes of Chloride, Protons, and Orthophosphate in Apical and Subhook Segments. Plant Physiol. 1979 Aug;64(2):211–213. doi: 10.1104/pp.64.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S. Proton Fluxes Associated with Sugar Uptake in Vicia faba Leaf Tissues. Plant Physiol. 1981 Sep;68(3):706–711. doi: 10.1104/pp.68.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank R. T., Badger M. R., Osmond C. B. Photosynthetic oxygen exchange in isolated cells and chloroplasts of c(3) plants. Plant Physiol. 1982 Oct;70(4):927–931. doi: 10.1104/pp.70.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S. Light-induced h secretion and the relation to senescence of oat leaves. Plant Physiol. 1982 Oct;70(4):1120–1124. doi: 10.1104/pp.70.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Role of o(2) and mitochondrial respiration in a photosynthetic stimulation of oat protoplast acidification of a surrounding medium. Plant Physiol. 1983 Jun;72(2):356–361. doi: 10.1104/pp.72.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Levine R. P. Reversible pH Changes in Cells of Chlamydomonas reinhardi Resulting from CO(2) Fixation in the Light and Its Evolution in the Dark. Plant Physiol. 1971 May;47(5):700–704. doi: 10.1104/pp.47.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Use of lipophilic cations to measure the membrane potential of oat leaf protoplasts. Plant Physiol. 1978 Dec;62(6):927–929. doi: 10.1104/pp.62.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P., Sharaf A. A., Liberman E. A. Proton conductors in the respiratory chain and artificial membranes. Nature. 1967 Nov 18;216(5116):718–719. doi: 10.1038/216718a0. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


