Abstract
Purpose
To explore the correlation between the initial recurrence site and survival after recurrence (PRS) in non-small cell lung cancer (NSCLC).
Methods
We collected 588 stages I–III NSCLC patients with recurrence after radical resection in Yunnan Cancer Hospital from January 2013 to December 2018. We used Kaplan–Meier survival curves to compare PRS in patients with different site recurrences. The univariate and multivariate Cox proportional hazard models were used to analyze the impact of the initial recurrence site on PRS.
Results
The recurrence site included the lung (n = 109), brain (n = 113), bone (n = 79), abdomen (n = 28), pleura (n = 24), lymph node (n = 81), and multisite (n = 154). In the total population, patients with multisite recurrence had substantially worse PRS (24.8 months, 95% confidence interval [CI]: 17.46–32.20) than that of patients without multiple sites recurrence (42.2 months, 95% CI 32.24–52.10) (P = 0.026). However, patients with lung recurrence had better RFS (63.1 months, 95% CI 51.13–74.00) than those who did not (31.0 months, 95% CI 25.10–36.96) (P < 0.001). In adenocarcinoma, patients with pleural recurrence had substantially worse PRS (21.3 months, 95% CI 15.07–27.46) than that of patients without pleural recurrence (46.9 months, 95% CI 35.07–58.80) (P = 0.031). Multivariate Cox proportional hazards regression analysis revealed that lung recurrence (HR 0.58, 95% CI 0.40–0.82; P = 0.003) was independent protective prognostic factor for PRS in the total population, while pleural recurrence (HR 2.18, 95% CI 1.14–4.17; P = 0.018) was independent adverse prognostic factors for PRS in adenocarcinoma patients.
Conclusion
The initial recurrence site was associated with PRS in NSCLC patients. Identification of recurrence sites could guide the subsequent treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03252-x.
Keywords: Non-small cell lung cancer, Site of initial recurrence, Post-recurrence survival, Prognostic
Introduction
Lung cancer has a high incidence and fatality rate globally [1]. Approximately, 40% of stages I–III non-small cell lung cancer (NSCLC) patients often recur after radical resection [2–5]. The prognosis following recurrence was poor, and less than 2 years have been recorded as survival following a recurrence of primary lung cancer [4–8]. Studies have also shown that postoperative chemotherapy and radiotherapy can improve the PRS of patients [8, 9]. However post-recurrence survival (PRS) varied significantly among different patients. The PRS was negatively impacted by males [4, 6, 7], older age [4, 7, 10], poor motor status [5], abdominal and bone metastases [7], and poor differentiation [6, 10, 11]. Identifying risk factors affecting survival after relapse could help clinicians to make the subsequent treatment regimen. A lot of studies have explored or predicted prognostic factors for relapse-free survival in non-small cell lung cancer [12–14]. However, little is known about the factors affecting PRS in NSCLC patients, and further studies are needed.
Few studies have explored the association of recurrence sites with PRS in NSCLC patients, particularly adenocarcinoma patients. Some studies show that patients with local recurrence, as opposed to distant recurrence, have a better PRS [13, 15]. However, in several studies, distant recurrence did not impact PRS [5, 6, 16]. Besides, some studies show that poor PRS was seen in patients with bone metastases [7, 9, 11], liver metastases [6, 8], lung metastases [4], and abdominal organ metastases [7]. The survival time of liver metastasis was 7.8 months [7]. Median survival with brain metastases was 7–10 months [17]. The more metastatic organs, the shorter the survival time, but there was no significant difference in the survival time of patients with three or four or more metastatic organs [17]. Inaccurate identification of the initial recurrence site is the reason for the poor prognosis of patients after recurrence. However, there is limited research on the effect of each recurrence site on postoperative prognosis. Furthermore, there is no consensus on which recurrence site has a poorer PRS when NSCLC or adenocarcinoma first recurs.
Hence, this study aimed to explore the correlation between the initial recurrence site and PRS in NSCLC patients; to find out which organ metastasis of non-small cell lung cancer has the worst prognosis, especially adenocarcinoma; and to guide clinical decision-making and improve patient prognosis.
Patients and methods
Ethics statement
The study protocol (KY2019141) was reviewed and approved by the Institutional Review Board of the Yunnan Cancer Hospital. It was carried out following the fundamental principles of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
Patients
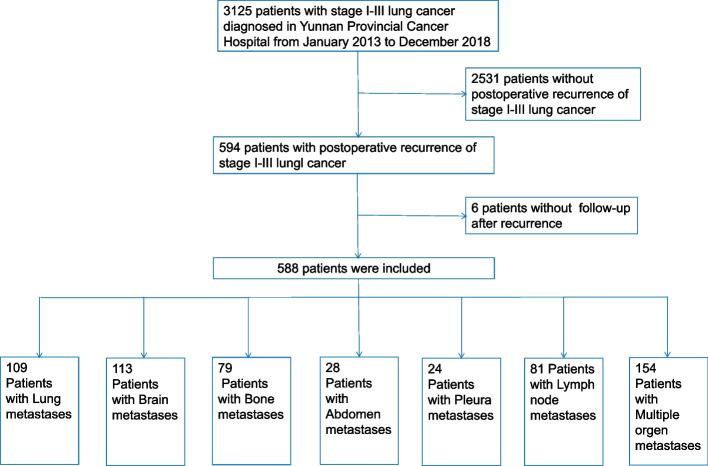
From January 2013 to December 2018, this retrospective study collected consecutively 3125 patients with pathologically confirmed stages I–III primary lung cancer in Yunnan Cancer Hospital after radical resection of lung cancer. Among them, 588 patients with recurrence were included in the analysis. A total of 2531 patients with recurrence and 6 patients who were lost to follow-up were excluded (Fig. 1).
Fig. 1.
Inclusion and exclusion flow charts
Observation following surgery and recurrence
Chest and abdomen CT and blood tumor markers were routinely performed every 3 to 6 months within 3 years after surgery, and checkups were performed every 6 to 12 months for 4–5 years after surgery. Imaging examinations were carried out following symptoms when symptoms emerged during follow-up: positron emission tomography (PET)-CT or bone scan for bone pain, magnetic resonance imaging (MRI) of the head for headaches, contrast-enhanced CT or MRI, abdominal ultrasound, or gastrointestinal endoscopy for abdominal pain. After the identification of recurrence, any further systemic treatment was at the discretion of the multidisciplinary team.
Initial recurrence organs classification and word meanings
The initial recurrence organs were divided into the following seven subgroups: the (i) lung, (ii) brain, (iii) bone, (iv) abdomen (liver and adrenal glands), (v) pleural, (vi) lymph node, and (vii) multisite (two or more organs). PRS in patients with relapsing sites was analyzed. PRS was defined as the time to all-cause death from the first time there was evidence of relapse to the last observation period in an event-free review patient.
Statistical analysis
For continuous variables, the t-test was employed. For categorical variables, the Fisher exact test was employed. Both univariate and multivariate analyses for PRS were conducted using the Cox proportional hazard model. The variables included age, sex, BMI, surgical modality, degree of differentiation, pathological stage of AJCC, vascular invasion, bronchial invasion, pleural invasion, recurrent sites (lung, brain, bone, abdomen, pleura, lymph nodes, multisite), two recurrent sites, three or more relapsed sites, adjuvant chemotherapy, radiotherapy, and targeted therapy. For variables in the univariate analysis with P < 0.05, multivariate analysis was carried out. The Kaplan–Meier method was employed for calculating PRS. The log-rank test was performed to compare groups. P < 0.05 is regarded as significant in statistical terms. R4.2.2 (R project) was used for statistical analysis.
Results
In the end, 588 patients were included in the study following the inclusion and exclusion criteria. The median age of patients was 58.00 (33.00–85.00), and the median BMI was 22.65 (6.38–68.89) (Table 1). There were 109 cases (18.54%) of lung recurrence, 113 cases (19.22%) of brain recurrence, 79 cases (13.44%) of bone recurrence, 28 cases (4.76%) of abdominal recurrence, 24 cases (4.08%) of pleural recurrence, 154 cases (26.19%) of multisite recurrence, 81 cases (13.78%) of lymph node recurrence, and 28 cases (4.76%) of abdominal recurrence. There were 14 cases of liver metastasis and 14 cases of adrenal metastasis among patients with recurrence of abdominal organs. The median PRS in the total population was 23.75 (range 0.03–104.97) months. The PRS for adenocarcinoma was 25.05 (0.07–104.97) months (Table 1).
Table 1.
Clinicopathological characteristics of patients
| Characteristic | All patients (n = 588) | Adenocarcinoma patients (n = 427) | Squamous carcinoma patients (n = 116) | Other patients4 (n = 45) | p-value6 |
|---|---|---|---|---|---|
|
Age (years) Mean (SD) Median (IQR) |
58.66 (9.32) 58.00 (33.00–85.00) |
57.85 (9.33) 57.00 (33.00–85.00) |
61.49 (8.38) 62.00 (42.00–83.00) |
59.07 (10.28) 59.00 (38.00–84.00) |
< 0.001 |
|
BMI1 (kg/m2) Mean (SD) Median (IQR) |
23.06 (3.71) 22.65 (6.38–68.89) |
23.10 (3.91) 22.83 (6.38–68.89) |
22.86 (3.11) 22.40 (14.86–31.49) |
23.14 (3.27) 22.38 (18.07–32.32) |
0.816 |
|
PRS (M) Mean (SD) Median (IQR) |
28.78 (22.03) 23.75 (0.03–104.97) |
30.01 (22.52) 25.05 (0.07–104.97) |
25.91 (20.38) 21.00 (0.03–102.40) |
24.62 (20.61) 17.82 (2.40–102.80) |
0.089 |
| Sex | < 0.001 | ||||
| Male | 359 (61.05%) | 213 (49.88%) | 113 (97.41%) | 33 (73.33%) | |
| Female | 229 (38.95%) | 214 (50.12%) | 3 (2.59%) | 12 (26.67%) | |
| Surgical mode | < 0.001 | ||||
| Lobectomy | 508 (86.39%) | 367 (85.95%) | 101 (87.07%) | 40 (88.89%) | |
| Segmentectomy | 6 (1.02%) | 6 (1.41%) | 0 (0.00%) | 0 (0.00%) | |
| Wedge resection | 54 (9.18%) | 50 (11.71%) | 2 (1.72%) | 2 (4.44%) | |
| Total pneumonectomy | 20 (3.40%) | 4 (0.94%) | 13 (11.21%) | 3 (6.67%) | |
| Tumor differentiation | < 0.001 | ||||
| Unknown | 377 (64.12%) | 329 (77.05%) | 16 (13.79%) | 32 (71.11%) | |
| Medium differentiation | 98 (16.67%) | 41 (9.60%) | 55 (47.41%) | 2 (4.44%) | |
| Low differentiation | 98 (16.67%) | 48 (11.24%) | 44 (37.93%) | 6 (13.33%) | |
| Undifferentiation | 10 (1.70%) | 4 (0.94%) | 1 (0.86%) | 5 (11.11%) | |
| Medium–low differentiation | 5 (0.85%) | 5 (1.17%) | 0 (0.00%) | 0 (0.00%) | |
| Vascular cancer thrombus | 0.820 | ||||
| Yes | 23 (3.91%) | 17 (3.98%) | 5 (4.31%) | 1 (2.22%) | |
| No | 565 (96.09%) | 410 (96.02%) | 111 (95.69%) | 44 (97.78%) | |
| Bronchial stump | 0.600 | ||||
| Yes | 38 (6.46%) | 25 (5.85%) | 9 (7.76%) | 4 (8.89%) | |
| No | 550 (93.54%) | 402 (94.15%) | 107 (92.24%) | 41 (91.11%) | |
| Pleural invasion | 0.158 | ||||
| Yes | 109 (18.83%) | 72 (17.06%) | 28 (25.00%) | 9 (20.00%) | |
| No | 470 (81.17%) | 350 (82.94%) | 84 (75.00%) | 36 (80.00%) | |
| AJCC1 8th ed. stage | 0.225 | ||||
| AICC < = II stage | 308 (52.38%) | 233 (54.57%) | 54 (46.55%) | 21 (46.67%) | |
| AICC > II stage | 280 (47.62%) | 194 (45.43%) | 62 (53.45%) | 24 (53.33%) | |
| Adjuvant chemotherapy | 0.019 | ||||
| Yes | 479 (81.46%) | 336 (78.69%) | 103 (88.79%) | 40 (88.89%) | |
| No | 109 (18.54%) | 91 (21.31%) | 13 (11.21%) | 5 (11.11%) | |
| Adjuvant radiation therapy | 0.002 | ||||
| Yes | 174 (29.59%) | 111 (26.00%) | 41 (35.34%) | 22 (48.89%) | |
| No | 414 (70.41%) | 316 (74.00%) | 75 (64.66%) | 23 (51.11%) | |
| Postoperative targeted therapy | 0.142 | ||||
| Yes | 45 (7.65%) | 38 (8.90%) | 4 (3.45%) | 3 (6.67%) | |
| No | 543 (92.35%) | 389 (91.10%) | 112 (96.55%) | 42 (93.33%) | |
| Two sites | 0.935 | ||||
| Yes | 81 (13.78%) | 58 (13.58%) | 16 (13.79%) | 7 (15.56%) | |
| No | 507 (86.22%) | 369 (86.42%) | 100 (86.21%) | 38 (84.44%) | |
| Three or more recurrence sites | 0.514 | ||||
| Yes | 64 (10.88%) | 46 (10.77%) | 15 (12.93%) | 3 (6.67%) | |
| No | 524 (89.12%) | 381 (89.23%) | 101 (87.07%) | 42 (93.33%) | |
| Lung recurrence | 0.030 | ||||
| Yes | 109 (18.54%) | 90 (21.08%) | 15 (12.93%) | 4 (8.89%) | |
| No | 479 (81.46%) | 337 (78.92%) | 101 (87.07%) | 41 (91.11%) | |
| Brain recurrence | 0.012 | ||||
| Yes | 113 (19.22%) | 93 (21.78%) | 11 (9.48%) | 9 (20.00%) | |
| No | 475 (80.78%) | 334 (78.22%) | 105 (90.52%) | 36 (80.00%) | |
| Bone recurrence | 0.172 | ||||
| Yes | 79 (13.44%) | 56 (13.11%) | 13 (11.21%) | 10 (22.22%) | |
| No | 509 (86.56%) | 371 (86.89%) | 103 (88.79%) | 35 (77.78%) | |
| Abdominal organs2 recurrence | 0.023 | ||||
| Yes | 28 (4.76%) | 14 (3.28%) | 10 (8.62%) | 4 (8.89%) | |
| No | 560 (95.24%) | 413 (96.72%) | 106 (91.38%) | 41 (91.11%) | |
| Pleural recurrence | 0.479 | ||||
| Yes | 24 (4.08%) | 15 (3.51%) | 6 (5.17%) | 3 (6.67%) | |
| No | 564 (95.92%) | 412 (96.49%) | 110 (94.83%) | 42 (93.33%) | |
| Lymph node5 recurrence | < 0.001 | ||||
| Yes | 81 (13.78%) | 46 (10.77%) | 30 (25.86%) | 5 (11.11%) | |
| No | 507 (86.22%) | 381 (89.23%) | 86 (74.14%) | 40 (88.89%) | |
| Multisite3 recurrence | 0.819 | ||||
| Yes | 154 (26.19%) | 113 (26.46%) | 31 (26.72%) | 10 (22.22%) | |
| No | 434 (73.81%) | 314 (73.54%) | 85 (73.28%) | 35 (77.78%) | |
1Abbreviations: BMI, body mass index; AJCC, American Joint Committee on Cancer. 2Abdominal organs (liver + adrenal). 3Multisite (two or more organs). 4Other patients (neuroendocrine carcinoma, adenosquamous cell carcinoma, carcinoid). 5Lymph node (hilar, supraclavicular, and thoracic lymph nodes). 6P-value, using Pearson’s chi-squared test. Wilcoxon rank-sum test. Fisher’s exact test
In the total population, in comparison to patients without multisite recurrence (42.2 months, 95% CI 32.24–52.10), patients with multisite recurrence (24.8 months, 95% CI 17.46–32.20) (P = 0.026) had substantially worse PRS (Figure S1G). However, patients who had lung recurrence (63.1 months, 95% CI 51.13–74.00) had better PRS than patients who did not (31.0 months, 95% 25.10–36.96) (P < 0.001) (Figure S1A). Patients with or without brain, bone, abdominal, pleural, and lymph node recurrence were not statistically significant (Figure S1).
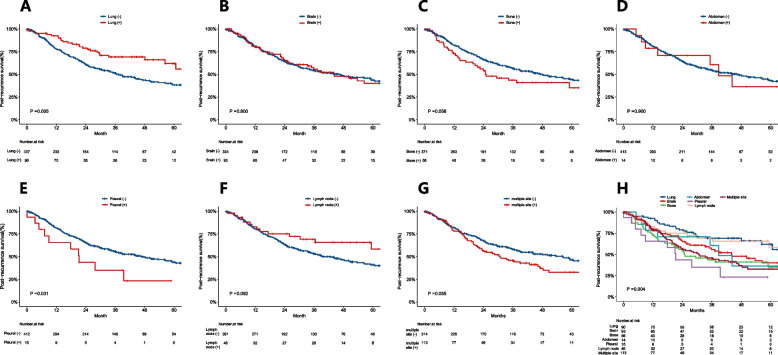
In adenocarcinoma, in comparison to patients without pleural recurrence (46.9 months, 95% CI 35.07–58.80), patients with pleural recurrence (21.3 months, 95% CI 15.07–27.46) (P = 0.031) had substantially worse PRS (Fig. 2E). However, patients who did lung recurrence (63.1 months, 95% CI 54.36–71.78) had better RFS than those who did not (37.3 months, 95% CI 29.0–45.60) (P = 0.005) (Fig. 2A). Patients with or without brain, bone, abdomen, lymph nodes, and multisite recurrence were not statistically significant (Fig. 2). In Fig. 2H, patients with pleural recurrence (21.3 months, 95% CI 15.07–27.46) (P = 0.004) had the worst PRS.
Fig. 2.
The PRS of patients with pleural recurrence was worse than that of patients without pleural recurrence (E), and lung recurrence is the opposite (A) in adenocarcinoma. There were no significant differences in the PRS of patients with or without recurrence at other sites (B–C and F–G). Among all recurrence sites, pleural recurrence has the worst prognosis (H). PRS, post-recurrence survival
In squamous cell carcinoma, in comparison to patients without multisite recurrence (30.0 months, 95% CI 21.84–38.16), patients with multisite recurrence (17.8 months, 95% CI 11.19–24.41; P = 0.038) had substantially worse PRS (Figure S2G). Patients with or without lung, brain, bone, abdomen, lymph nodes, and pleural recurrence were not statistically significant (Figure S2).
In multivariate analysis of the general population, lung recurrence was a protected factor for PRS (HR 0.58, 95% CI 0.40–0.82; P = 0.003), and female, BMI (≥ 24), vascular invasion, AICC > II stage, and three or more recurrent sites were poor prognostic factors for PRS (Table S1).
In multivariate analysis of the adenocarcinoma, lung recurrence was a protective factor for PRS (HR 0.62, 95% CI 0.41–0.95; P = 0.027), and pleural recurrence (HR 2.18, 95% CI 1.14–4.17; P = 0.018), vascular invasion, AICC > II stage, and three or more recurrent sites were poor prognostic factors for PRS (Table 2). In multivariate analysis of the squamous cell carcinoma, older age (≥ 60 years) and three or more recurrence sites were poor prognostic factors for PRS (Table S2).
Table 2.
Univariate and multivariate analysis of recurrence survival for adenocarcinoma
| Post-recurrence survival variable | Univariate HR1 (95% CI1) |
p-value5 | Multivariate HR1 (95% CI1) |
p-value5 |
|---|---|---|---|---|
| Sex | ||||
| Male | 1.0 (reference) | |||
| Female | 0.88 (0.66, 1.16) | 0.363 | ||
| Age group | ||||
| < 60 | 1.0 (reference) | |||
| ≥ 60 | 0.98 (0.73, 1.30) | 0.884 | ||
| BMI1 group | ||||
| < 24 | 1.0 (reference) | |||
| ≥ 24 | 0.74 (0.55, 1.00) | 0.052 | ||
| Surgical mode | ||||
| Lobectomy | 1.0 (reference) | |||
| Segmentectomy | 0.29 (0.04, 2.05) | 0.213 | ||
| Wedge resection | 1.44 (0.96, 2.16) | 0.081 | ||
| Total pneumonectomy | 1.29 (0.32, 5.20) | 0.722 | ||
| Tumor differentiation | ||||
| Unknown | 1.0 (reference) | |||
| Medium differentiation | 0.85 (0.52, 1.39) | 0.522 | ||
| Low differentiation | 1.19 (0.78, 1.80) | 0.420 | ||
| Undifferentiation | 0.50 (0.07, 3.57) | 0.488 | ||
| Medium–low differentiation | 1.88 (0.60, 5.89) | 0.281 | ||
| Vascular cancer thrombus | ||||
| No | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 1.93 (1.08, 3.47) | 0.027 | 2.00 (1.11, 3.61) | 0.021 |
| Bronchial stump | ||||
| No | 1.0 (reference) | |||
| Yes | 1.62 (0.98, 2.67) | 0.058 | ||
| Pleural invasion | ||||
| No | 1.0 (reference) | |||
| Yes | 0.85 (0.56, 1.28) | 0.427 | ||
| AJCC1 8th ed. stage | ||||
| AICC < = II stage | 1.0 (reference) | 1.0 (reference) | ||
| AICC > II stage | 1.78 (1.34, 2.37) | < 0.001 | 1.70 (1.27, 2.27) | < 0.001 |
| Adjuvant chemotherapy | ||||
| No | 1.0 (reference) | |||
| Yes | 0.87 (0.61, 1.22) | 0.415 | ||
| Adjuvant radiation therapy | ||||
| No | 1.0 (reference) | |||
| Yes | 0.89 (0.65, 1.22) | 0.474 | ||
| Postoperative targeted therapy | ||||
| No | 1.0 (reference) | |||
| Yes | 0.97 (0.61, 1.54) | 0.891 | ||
| Two sites | ||||
| No | 1.0 (reference) | |||
| Yes | 0.96 (0.63, 1.45) | 0.839 | ||
| Three or more recurrence sites | ||||
| No | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 1.80 (1.22, 2.66) | 0.003 | 1.60 (1.07, 2.39) | 0.022 |
| Lung recurrence | ||||
| No | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 0.56 (0.37, 0.84) | 0.005 | 0.62 (0.41, 0.95) | 0.027 |
| Brain recurrence | ||||
| No | 1.0 (reference) | |||
| Yes | 0.96 (0.68, 1.35) | 0.804 | ||
| Bone recurrence | ||||
| No | 1.0 (reference) | |||
| Yes | 1.44 (0.99, 2.09) | 0.058 | ||
| Abdominal organs2 recurrence | ||||
| No | 1.0 (reference) | |||
| Yes | 1.05 (0.49, 2.24) | 0.896 | ||
| Pleural recurrence | ||||
| No | 1.0 (reference) | 1.0 (reference) | ||
| Yes | 1.99 (1.05, 3.77) | 0.034 | 2.18 (1.14, 4.17) | 0.018 |
| Lymph node4 recurrence | ||||
| No | 1.0 (reference) | |||
| Yes | 0.64 (0.38, 1.08) | 0.094 | ||
| Multisite3 recurrence | ||||
| No | 1.0 (reference) | |||
| Yes | 1.35 (0.99, 1.83) | 0.057 | ||
1Abbreviations: BMI, body mass index; AJCC, American Joint Committee on Cancer; CI, confidence interval; HR, hazard ratio. 2Abdominal organs (liver + adrenal). 3Multisite (two or more organs). 4Lymph node (hilar, supraclavicular, and thoracic lymph nodes). 5P-value, using Pearson’s chi-squared test. Wilcoxon rank-sum test. Fisher’s exact test
Discussion
This study found that lung recurrence was an independent prognostic factor for PRS in the general population. Still, brain recurrence, bone recurrence, abdominal recurrence, pleural recurrence, lymph node recurrence, and multiple site recurrence did not affect PRS. In adenocarcinoma, lung and pleural recurrence were independent prognostic factors for PRS. In contrast, brain recurrence, bone recurrence, abdominal recurrence, lymph node recurrence, and multiple site recurrence did not affect PRS. It was not found which single-organ recurrence was an independent prognostic factor for PRS in squamous cell carcinoma.
Previous studies have reported that recurrence at two or more sites does not affect the prognosis of lung cancer [7]. However, in this study, we divided them into two subgroups: two recurrence sites and three or more recurrence sites. Therefore, multivariate analysis showed three or more recurrence sites with statistical significance and poor prognosis. In comparison, two recurrence sites were not statistically significant in the general population, adenocarcinoma, and squamous cell carcinoma.
Different studies have found different prognoses of PRS at various recurrence sites, such as liver recurrence. Some studies have shown that RFS in patients with abdominal organ recurrence is significantly worse and frequently recurrent [7, 18]. Additionally, it has been reported that the prognosis was poor when the liver was the first abdominal organ to recur following lung cancer resection [5, 8]; according to earlier research, patients with advanced lung cancer liver metastases also had a poor prognosis due to chemotherapy [19], TKI therapy [7, 18], immunological tolerance [20], and quicker tumor growth [21]. Nevertheless, some studies have shown that liver metastases do not influence PRS [7, 9, 11].
Different investigations have found other effects of lung recurrence on PRS prognosis. One study showed that patients with lung recurrence [4, 8] had better PRS, possibly because the lung recurrence was less malignant, which was also confirmed in our study. Moreover, radiotherapy and metastasectomy at lung recurrence sites may improve PRS in patients with lung recurrence [22, 23]. However, some research shows lung recurrence has little impact on PRS [6, 9, 11].
Some studies reported that pleural recurrence has nothing to do with PRS among lung cancer patients [7]. However, they did not do a subgroup analysis. Our study found through subgroup analysis that pleural recurrence was not statistically significant in the general population and squamous cell carcinoma in our study. Our finding was consistent with previous findings [7]. However, in adenocarcinoma, pleural recurrence patients have a poorer prognosis, possibly because there are few studies on pleural metastasis in lung cancer. A larger cohort may be needed to investigate further prognostic factors affecting pulmonary adenocarcinoma pleural recurrence to improve PRS in patients with adenocarcinoma pleural recurrence.
This study belongs to a large cohort study. For the first time, the impact of initial recurrence organs on lung cancer PRS was divided into the total population, adenocarcinoma, and squamous cell carcinoma to study separately, and the initial recurrence organs were divided into seven subgroups. There were a few limitations to this study. First, because this study was retrospective and only involved one institution, selection bias could not be ruled out; second, the method of postoperative monitoring was different for each doctor. Third, this study included fewer squamous cell carcinoma patients (n = 116), and the subsequent research could consist of more squamous cell carcinoma patients to analyze which initial recurrence site had the most significant effect on PRS in their population.
In conclusion, the initial recurrence site was associated with PRS in NSCLC patients. Identification of recurrence sites could guide the subsequent treatment. In adenocarcinoma, patients with pleural recurrence have the worst prognosis, which should be followed up as soon as possible, and early intervention treatment to improve the prognosis of patients. Patients of squamous cell carcinoma should pay more attention to multisite recurrence.
Supplementary Information
Additional file 1: Figure S1. The PRS of patients with lung and multiple site recurrence was worse than that of patients without lung and multiple site recurrence(G) and lung recurrence is the opposite in adenocarcinoma (A) in the total population. There were no significant differences in the PRS of patients with or without recurrence at other sites (B-F). Among all recurrence sites, lung recurrence has the best prognosis(H).PRS: post-recurrence survival.
Additional file 2: Figure S2. The PRS of patients with multiple site recurrence was worse than that of patients without multiple site recurrence in squamous cell carcinoma (G). There were no significant differences in the PRS of patients with or without recurrence at other sites (A-F, H).PRS: post-recurrence survival.
Additional file 3: Table S1. Univariate and multivariate analyses of post-recurrence survival in the general population.
Additional file 4: Table S2. Univariate and multivariate analysis of recurrence survival in squamous cell carcinoma.
Abbreviations
- CI
Confidence interval
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- HR
Hazard ratio
- MRI
Magnetic resonance imaging
- NSCLC
Non-small cell lung cancer
- PRS
Post-recurrence survival
- RFS
Relapse-free survival
- TKI
Tyrosine kinase inhibitor
- BMI
Body mass index
Authors’ contributions
Conception and design, LYL, CXB, LZH, and PHJ. Acquisition, analysis, or interpretation of data, LYL, LLZ, and YRM. Drafting of the manuscript, LYL and PHJ. Critical revision of the manuscript for important intellectual content, LYL, LZH, PHJ, and CXB. Statistical analysis, LYL, JZJ, and LQW. Administrative, technical, or material support, CXB, LZH, and PHJ. Study supervision, LYL, LZH, PHJ, and CXB. Final approval of the manuscript, all authors. We thank Yunnan Cancer Center for providing valuable data resources for this research. We are grateful to all the staff who participated in the data collection, thanks for their active cooperation and firm support.
Funding
This work was supported by the National Natural Scientific Foundation of China (82001986, 82360345), the Outstanding Youth Science Foundation of Yunnan Basic Research Project (202101AW070001), the Yunnan Basic Research Project (202201AT070010, 202301AT070187), and Innovation Team of Kunming Medical University (CXTD202110).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because they are related to patients but are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital & Yunnan Cancer Center) before the commencement of the research. All participants had implemented informed consent and signed the informed consent before the investigation. We confirm that all methods (diagnosis, staging, and treatment of lung cancer) were carried out by the “lung cancer clinical practice guidelines” from the National Comprehensive Cancer Network (NCCN) of the USA. The methods in this study were followed by the relevant guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanli Li, Lizhu Liu and Ruiming You contributed equally to this work.
Contributor Information
Hongjiang Pu, Email: puhongjiang@qq.com.
Zhenhui Li, Email: lizhenhui621@kmmu.edu.cn.
Xiaobo Chen, Email: chenxiaobo82@126.com.
References
- 1.Tanriverdi O, Alkan A, Ozseker B, Solak-Ozseker H, Kilinc RM. Synchronous duodenum and descending colon metastasis from primary lung neuroendocrine small-cell carcinoma: a case report and review of the literature. J Oncol Pharm Pract. 2020;26:1524–1529. doi: 10.1177/1078155220904133. [DOI] [PubMed] [Google Scholar]
- 2.Isaka T, Nakayama H, Ito H, Yokose T, Yamada K, Masuda M. Impact of the epidermal growth factor receptor mutation status on the prognosis of recurrent adenocarcinoma of the lung after curative surgery. BMC Cancer. 2018;18:959. doi: 10.1186/s12885-018-4849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo C, Sakurada A, Notsuda H, Noda M, Hoshikawa Y, Okada Y, Kondo T. Results of long-term follow-up of patients with completely resected non-small cell lung cancer. Ann Thorac Surg. 2012;93:1061–1068. doi: 10.1016/j.athoracsur.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, Sugimachi K. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7:204–209. [PubMed] [Google Scholar]
- 5.Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, Williams BA, Pairolero PC. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007; 83:409–417; discussioin 417–408. [DOI] [PubMed]
- 6.Shimada Y, Saji H, Yoshida K, Kakihana M, Honda H, Nomura M, Usuda J, Kajiwara N, Ohira T, Ikeda N. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest. 2013;143:1626–1634. doi: 10.1378/chest.12-1717. [DOI] [PubMed] [Google Scholar]
- 7.Isaka T, Ito H, Nakayama H, Yokose T, Saito H, Masuda M. Impact of the initial site of recurrence on prognosis after curative surgery for primary lung cancer. Eur J Cardiothorac Surg. 2022;61:778–786. doi: 10.1093/ejcts/ezab442. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34:499–504. doi: 10.1016/j.ejcts.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Kubouchi Y, Kidokoro Y, Ohno T, Yurugi Y, Wakahara M, Haruki T, Nakamura H. Prognostic factors for post recurrence survival in resected pathological stage I non-small cell lung cancer. Yonago Acta Med. 2017;60:213–219. doi: 10.33160/yam.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, Huang J, Travis WD, Rizk NP, Rudin CM, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33:2877–2884. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Wu Y, Shao J, Liu D, Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020;20:150. doi: 10.1186/s12885-020-6621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters BA, Hayes RB, Goparaju C, Reid C, Pass HI, Ahn J. The microbiome in lung cancer tissue and recurrence-free survival. Cancer Epidemiol Biomark Prev. 2019;28:731–740. doi: 10.1158/1055-9965.EPI-18-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong M, Lim YJ. Chronic hyperglycemia is an adverse prognostic factor for locoregional recurrence-free survival in small cell lung cancer patients treated with radical radiotherapy. Thoracic Cancer. 2022;13:2633–2640. doi: 10.1111/1759-7714.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Zeng H, Zhang G, Li R, Yuan Z, Ren J, Huang Y, Ren F, Zhang H, Fei K, et al. Development and validation of a nomogram based on preoperative variables for predicting recurrence‐free survival in stage IA lung adenocarcinoma. Thorac Cancer. 2023;14:3108–18. [DOI] [PMC free article] [PubMed]
- 15.Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22:156–161. doi: 10.1016/j.suronc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuguma H, Nakahara R, Wakamatsu I, Kishikawa T, Sugiyama T, Nakamura Y, Kasai T, Kamiyama Y, Hoshi N, Inoue K, et al. Definitive local therapy for oligo-recurrence in patients with completely resected non-small cell lung cancer. Am J Clin Oncol. 2020;43:210–217. doi: 10.1097/COC.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 17.Oh Y, Taylor S, Bekele BN, Debnam JM, Allen PK, Suki D, Sawaya R, Komaki R, Stewart DJ, Karp DD. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer. 2009;115:2930–2938. doi: 10.1002/cncr.24333. [DOI] [PubMed] [Google Scholar]
- 18.Wu KL, Tsai MJ, Yang CJ, Chang WA, Hung JY, Yen CJ, Shen CH, Kuo TY, Lee JY, Chou SH, et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88:187–194. doi: 10.1016/j.lungcan.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sridhar S, Paz-Ares L, Liu H, Shen K, Morehouse C, Rizvi N, Segal NH, Jin X, Zheng Y, Narwal R, et al. Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clin Lung Cancer. 2019;20:e601–e608. doi: 10.1016/j.cllc.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Okubo K, Bando T, Miyahara R, Sakai H, Shoji T, Sonobe M, Fujinaga T, Sato K, Wada H, Tanaka T. Resection of pulmonary metastasis of non-small cell lung cancer. J Thorac Oncol. 2009;4:203–207. doi: 10.1097/JTO.0b013e3181949c6a. [DOI] [PubMed] [Google Scholar]
- 23.Walsh GL, O'Connor M, Willis KM, Milas M, Wong RS, Nesbitt JC, Putnam JB, Jr., Lee JJ, Roth JA. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg 1995, 60:1563–1570; discussion 1570–1562. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The PRS of patients with lung and multiple site recurrence was worse than that of patients without lung and multiple site recurrence(G) and lung recurrence is the opposite in adenocarcinoma (A) in the total population. There were no significant differences in the PRS of patients with or without recurrence at other sites (B-F). Among all recurrence sites, lung recurrence has the best prognosis(H).PRS: post-recurrence survival.
Additional file 2: Figure S2. The PRS of patients with multiple site recurrence was worse than that of patients without multiple site recurrence in squamous cell carcinoma (G). There were no significant differences in the PRS of patients with or without recurrence at other sites (A-F, H).PRS: post-recurrence survival.
Additional file 3: Table S1. Univariate and multivariate analyses of post-recurrence survival in the general population.
Additional file 4: Table S2. Univariate and multivariate analysis of recurrence survival in squamous cell carcinoma.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are related to patients but are available from the corresponding author on request.