Abstract
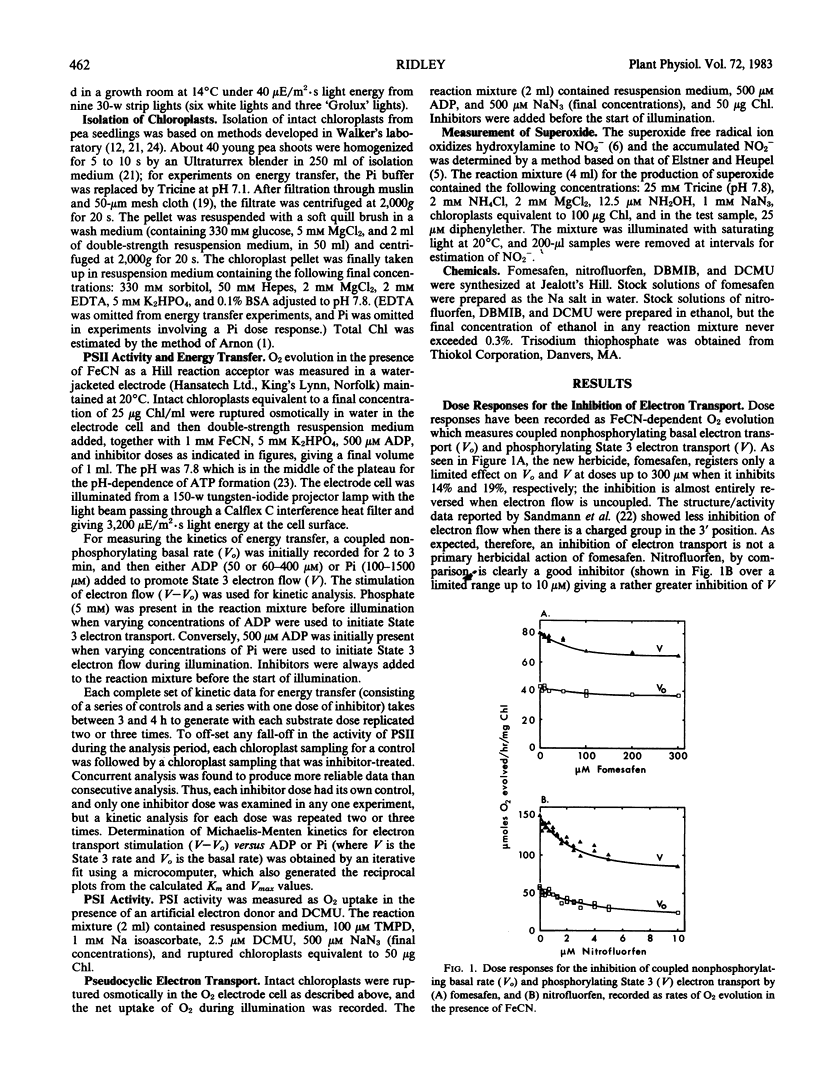
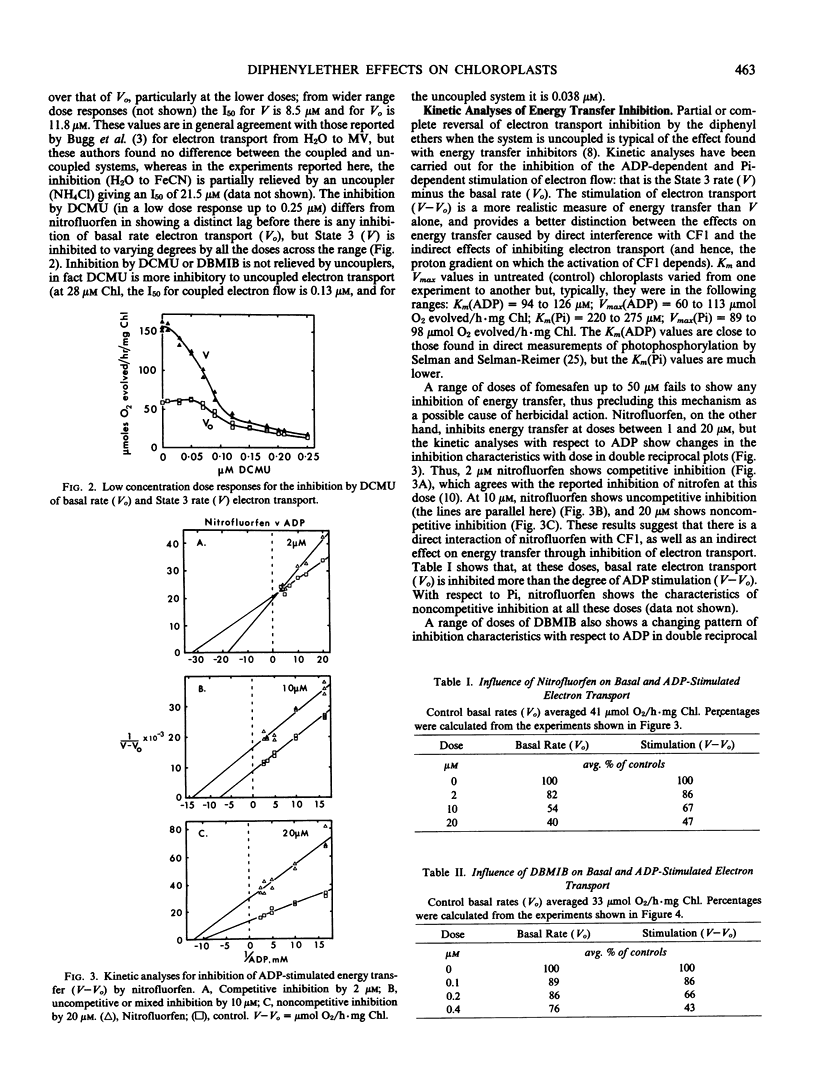
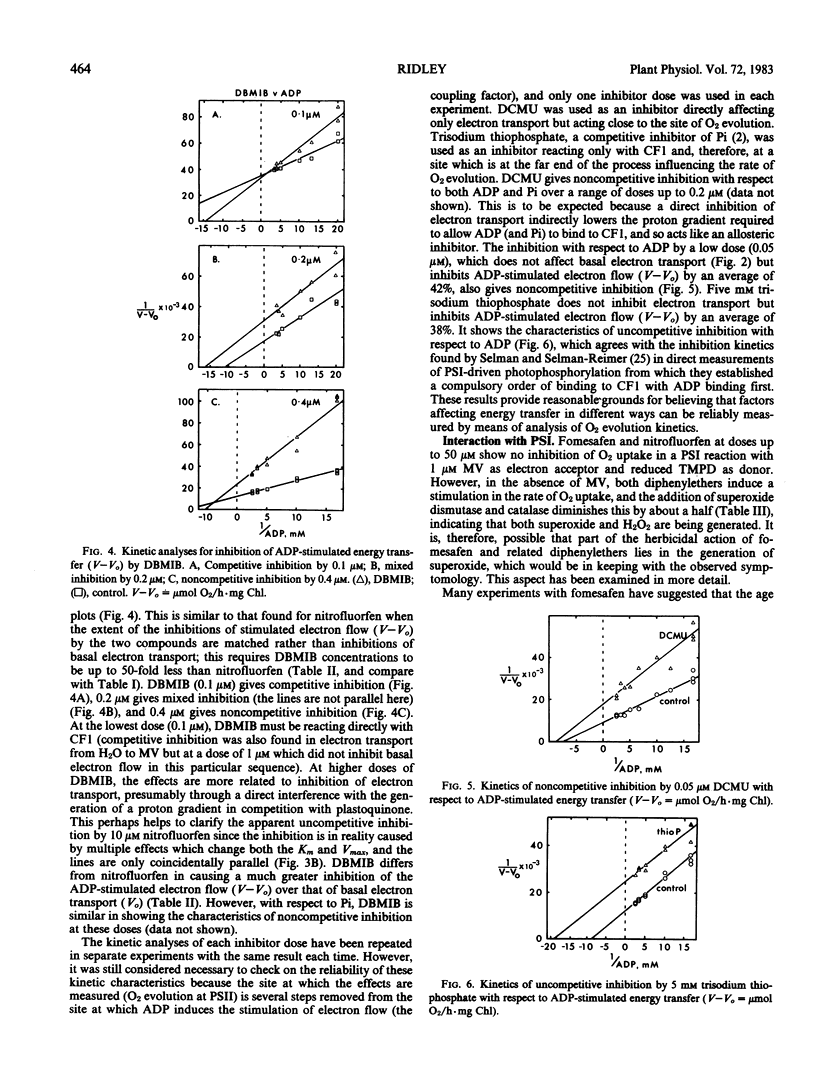
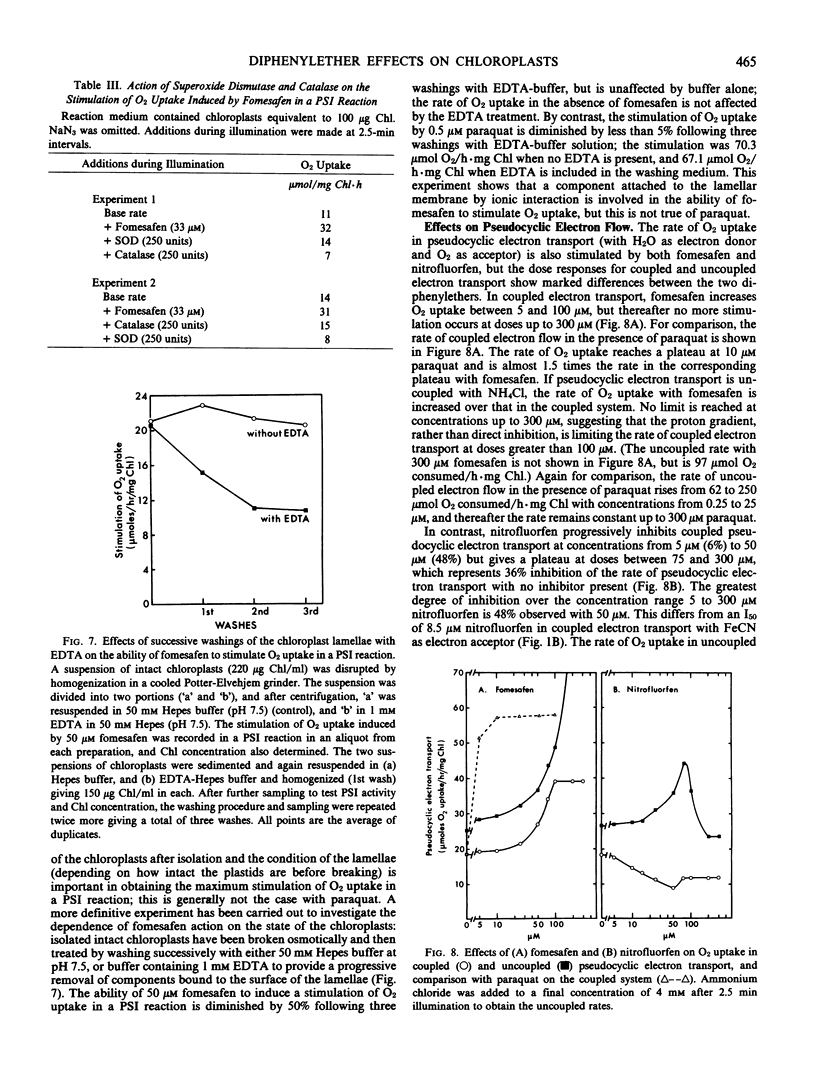
Several effects on pea (Pisum sativum L. var Onwards) chloroplasts of a new diphenylether herbicide, fomesafen (5-[2-chloro-4-trifluoromethyl-phenoxy]-N-methanesulfonyl-2 -nitrobenzamide) have been compared with those of a herbicide of related structure, nitrofluorfen (2-chloro-1-[4-nitrophenoxy]-4-[trifluoromethyl]benzene). Although both compounds produce the same light-dependent symptoms of desiccation and chlorosis indicative of a common primary mechanism of action, this study is concerned with a more broadly based investigation of different effects on the electron transport system. Comparisons have also been made with other compounds interacting with the chloroplast. Unlike nitrofluorfen, fomesafen has little effect as an inhibitor of electron flow or energy transfer. Both compounds have the ability to stimulate superoxide production through a functional electron transport system, and this involves specifically the p-nitro substituent. The stimulation, which is not likely to be an essential part of the primary herbicidal effect, is diminished under conditions that remove the coupling factor. Evidence suggests that both diphenylethers may be able to bind to the coupling factor, and kinetic studies reveal this for dibromothymoquinone as well. Such a binding site might be an important feature in allowing the primary effect of the diphenylether herbicides to be expressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Bugg M. W., Whitmarsh J., Rieck C. E., Cohen W. S. Inhibition of photosynthetic electron transport by diphenyl ether herbicides. Plant Physiol. 1980 Jan;65(1):47–50. doi: 10.1104/pp.65.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976 Feb;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Malkin R. Interaction of photosynthetic electron transport inhibitors and the Rieske Iron-Sulfur center in chloroplasts and the cytochrome b6-f complex. Biochemistry. 1982 Jun 8;21(12):2945–2950. doi: 10.1021/bi00541a022. [DOI] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. M. Interaction of chloroplasts with inhibitors: induction of chlorosis by diuron during prolonged illumination in vitro. Plant Physiol. 1977 Apr;59(4):724–732. doi: 10.1104/pp.59.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. The control of 3-phosphoglycerate reduction in isolated chloroplasts by the concentrations of ATP, ADP and 3-phosphoglycerate. Biochim Biophys Acta. 1979 Mar 15;545(3):528–536. doi: 10.1016/0005-2728(79)90161-0. [DOI] [PubMed] [Google Scholar]
- Schwenn J. D., Lilley R. M., Walker D. A. Inorganic pyrophospatase and photosynthesis by isolated chloroplasts. I. Characterisation of chloroplast pyrophosphatase and its relation to the response to exogenous pyrophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):586–595. doi: 10.1016/0005-2728(73)90218-1. [DOI] [PubMed] [Google Scholar]
- Selman B. R., Selman-Reimer S. The steady state kinetics of photophosphorylation. J Biol Chem. 1981 Feb 25;256(4):1722–1726. [PubMed] [Google Scholar]


