Abstract
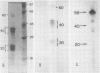
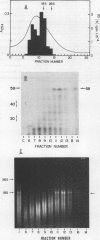
When polyadenylated RNA, isolated from membrane-bound polysomes extracted from developing oat (Avena sativa L.) seeds, was translated in vitro in the rabbit reticulocyte system, two polypeptides of about 58 and 60 kilodaltons were immunoprecipitated by anti-oat globulin antibody. No electrophoretic bands corresponding to the 40 and 20 kilodalton polypeptides of oat globulin were present. However, when in vivo labeled extracts were immunoprecipitated with anti-oat globulin antibody, three groups of polypeptides (60, 40, and 20 kilodaltons) were present. It therefore seems probable that the two large polypeptides (58 and 60 kilodaltons) were precursors of the 40 and 20 kilodalton polypeptides. When the polyadenylated RNA coding for these polypeptides was size fractionated on a sucrose density gradient, it sedimented near the 18S region of the gradient. Translation of the RNA from the gradient fractions and immunoprecipitation of translation products indicated that the template for the 58 to 60 kilodalton `putative' precursors of oat globulin was probably the RNA which was approximately 18S in size.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Separation and characterization of oat globulin polypeptides. Arch Biochem Biophys. 1982 Nov;219(1):71–79. doi: 10.1016/0003-9861(82)90135-7. [DOI] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Synthesis of Oat Globulin Precursors : Analogy to Legume 11S Storage Protein Synthesisa. Plant Physiol. 1982 Dec;70(6):1767–1769. doi: 10.1104/pp.70.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereken-Tumer N., Richter J. D., Nielsen N. C. Structural characterization of the glycinin precursors. J Biol Chem. 1982 Apr 25;257(8):4016–4018. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. M. Subunit structure and composition of oat seed globulin. Plant Physiol. 1978 Oct;62(4):506–509. doi: 10.1104/pp.62.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Hermodson M. A., Nielsen N. C. Identification of the acidic and basic subunit complexes of glycinin. J Biol Chem. 1981 Aug 25;256(16):8752–8755. [PubMed] [Google Scholar]
- Tumer N. E., Thanh V. H., Nielsen N. C. Purification and characterization of mRNA from soybean seeds. Identification of glycinin and beta-conglycinin precursors. J Biol Chem. 1981 Aug 25;256(16):8756–8760. [PubMed] [Google Scholar]