Abstract
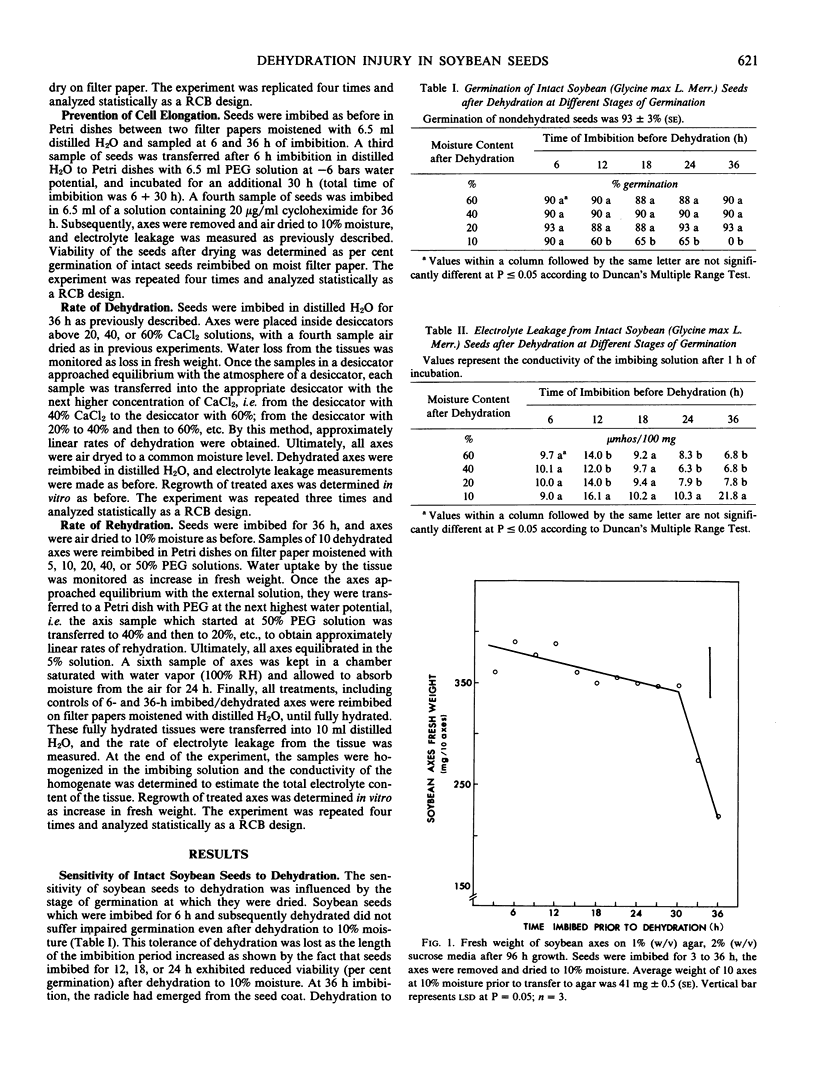
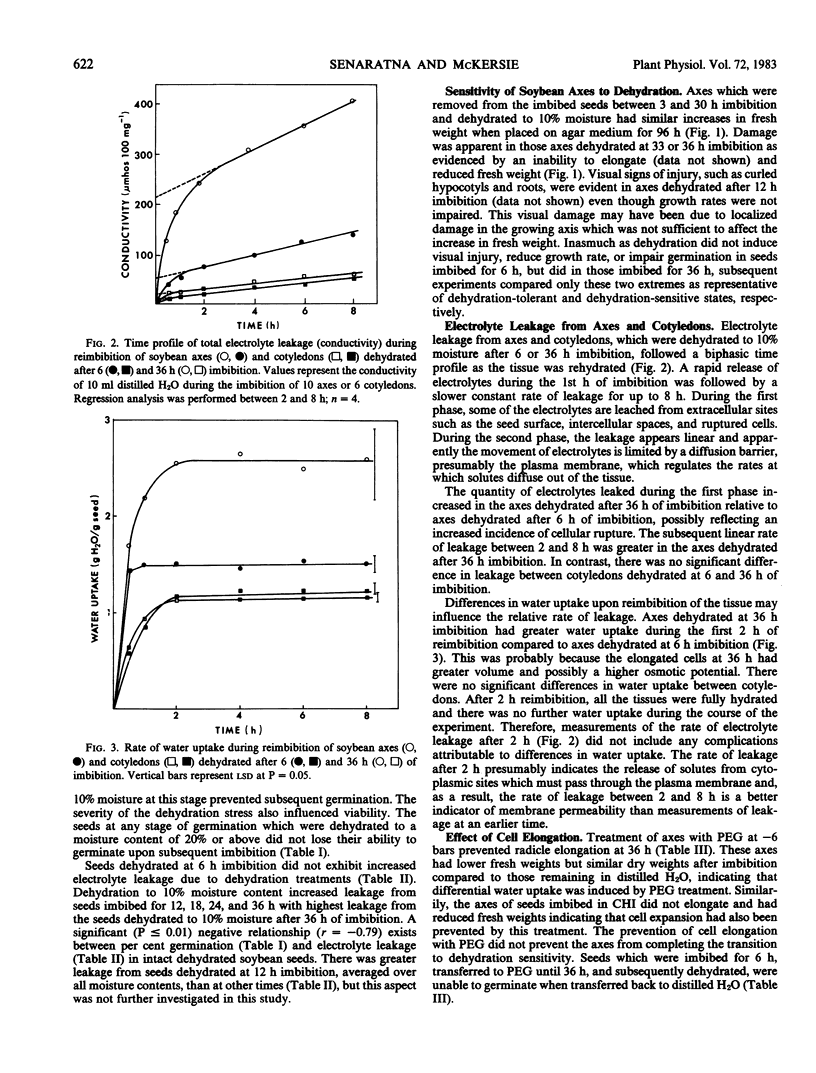
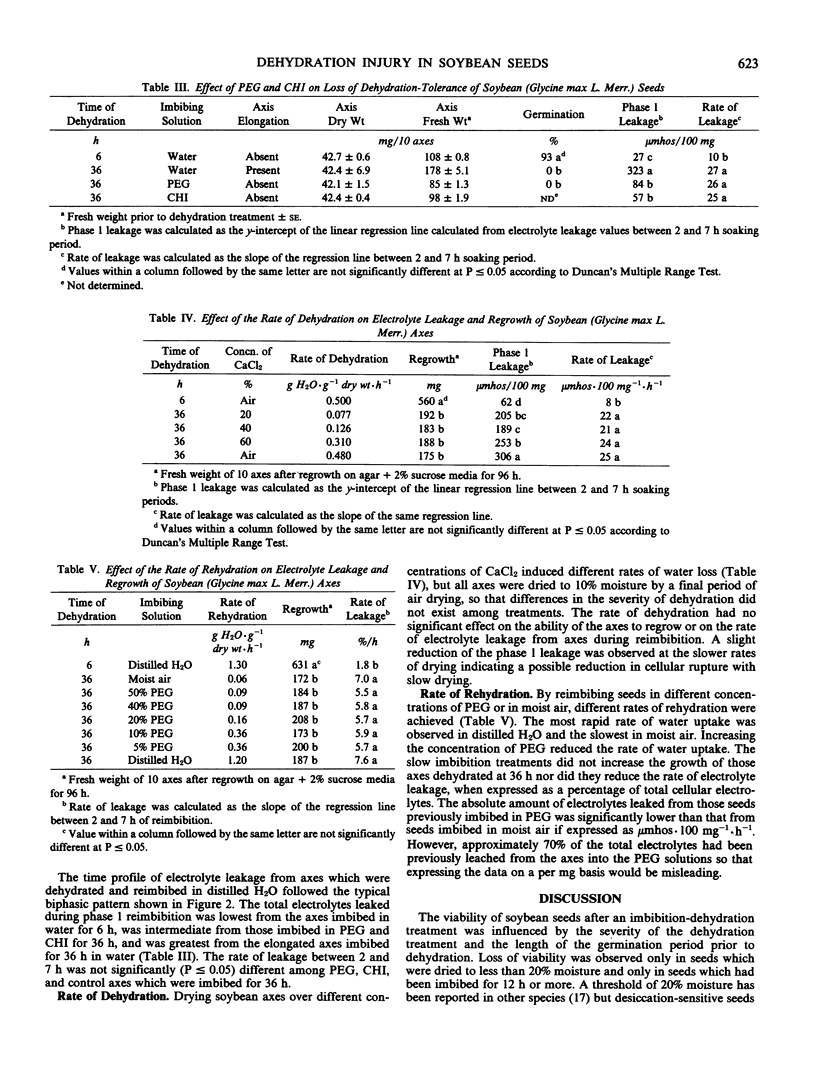
The sensitivity of soybean (Glycine max L. Merr. cv Maple Arrow) seeds to dehydration changed during germination. Seeds were tolerant of dehydration to 10% moisture if dried at 6 hours of imbibition, but were susceptible to dehydration injury if dried at 36 hours of imbibition. Dehydration injury appeared as loss of germination, slower growth rates of isolated axes, hypocotyl and root curling, and altered membrane permeability. Increased electrolyte leakage due to dehydration treatment was observed only from isolated axes but not from cotyledons, suggesting that cotyledons are more tolerant of dehydration. The transition from a dehydration-tolerant to a dehydration-susceptible state coincided with radicle elongation. However, the prevention of cell elongation by osmotic treatment in polyethylene glycol (−6 bars) or imbibition in 20 micrograms per milliliter cycloheximide did not prevent the loss of dehydration tolerance suggesting that neither cell elongation nor cytoplasmic protein synthesis was responsible for the change in sensitivity of soybean seeds to dehydration. Furthermore, the rate of dehydration or rate of rehydration did not alter the response to the dehydration stress.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becwar M. R., Stanwood P. C., Roos E. E. Dehydration effects on imbibitional leakage from desiccation-sensitive seeds. Plant Physiol. 1982 May;69(5):1132–1135. doi: 10.1104/pp.69.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Sarid S., Katchalski E. The role of water stress in the inactivation of messenger RNA of germinating wheat embryos. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1378–1383. doi: 10.1073/pnas.61.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Craft C. C., Audia W. V., Wilcox M. S. Biochemical Studies of Chilling Injury in Sweetpotatoes. Plant Physiol. 1958 Sep;33(5):307–311. doi: 10.1104/pp.33.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A., Weeks D. P., Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA in wheat embryo. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1681–1687. doi: 10.1073/pnas.67.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meryman H. T. Freezing injury and its prevention in living cells. Annu Rev Biophys Bioeng. 1974;3(0):341–363. doi: 10.1146/annurev.bb.03.060174.002013. [DOI] [PubMed] [Google Scholar]
- Volger H., Heber U., Berzborn R. J. Loss of function of biomembranes and solubilization of membrane proteins during freezing. Biochim Biophys Acta. 1978 Aug 17;511(3):455–469. doi: 10.1016/0005-2736(78)90281-x. [DOI] [PubMed] [Google Scholar]
- Wiest S. C., Steponkus P. L. Freeze-thaw injury to isolated spinach protoplasts and its simulation at above freezing temperatures. Plant Physiol. 1978 Nov;62(5):699–705. doi: 10.1104/pp.62.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]


