Abstract
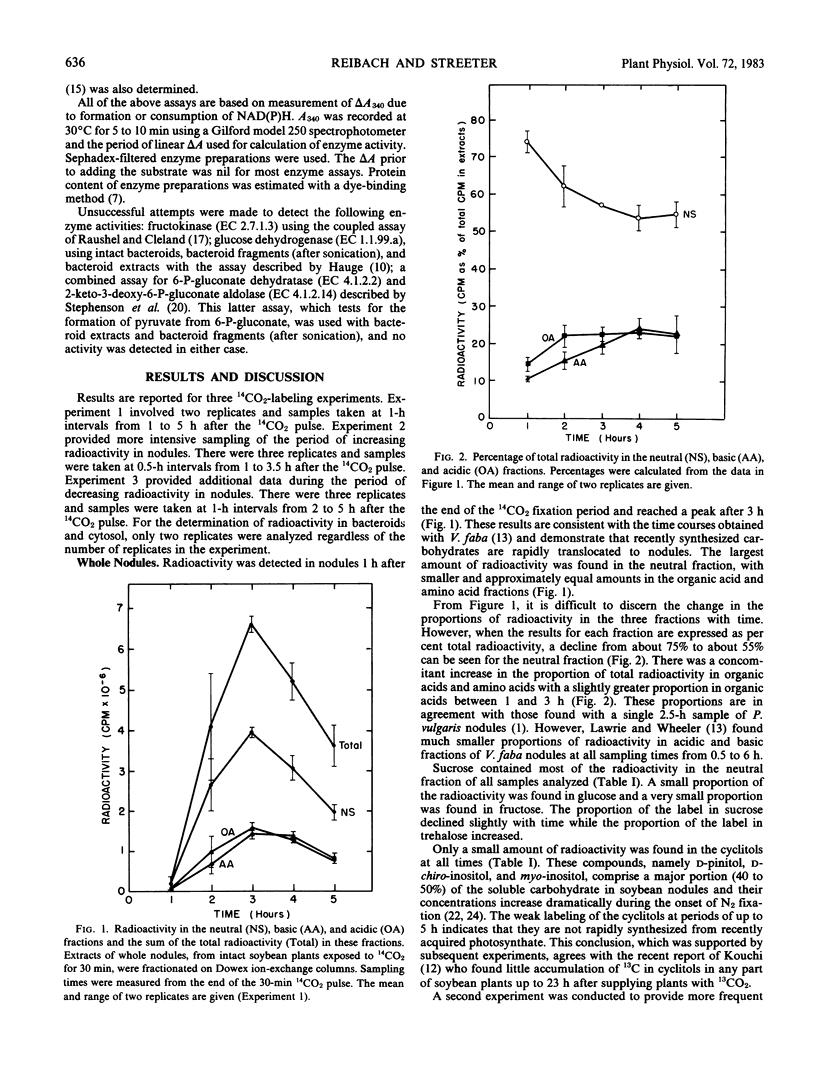
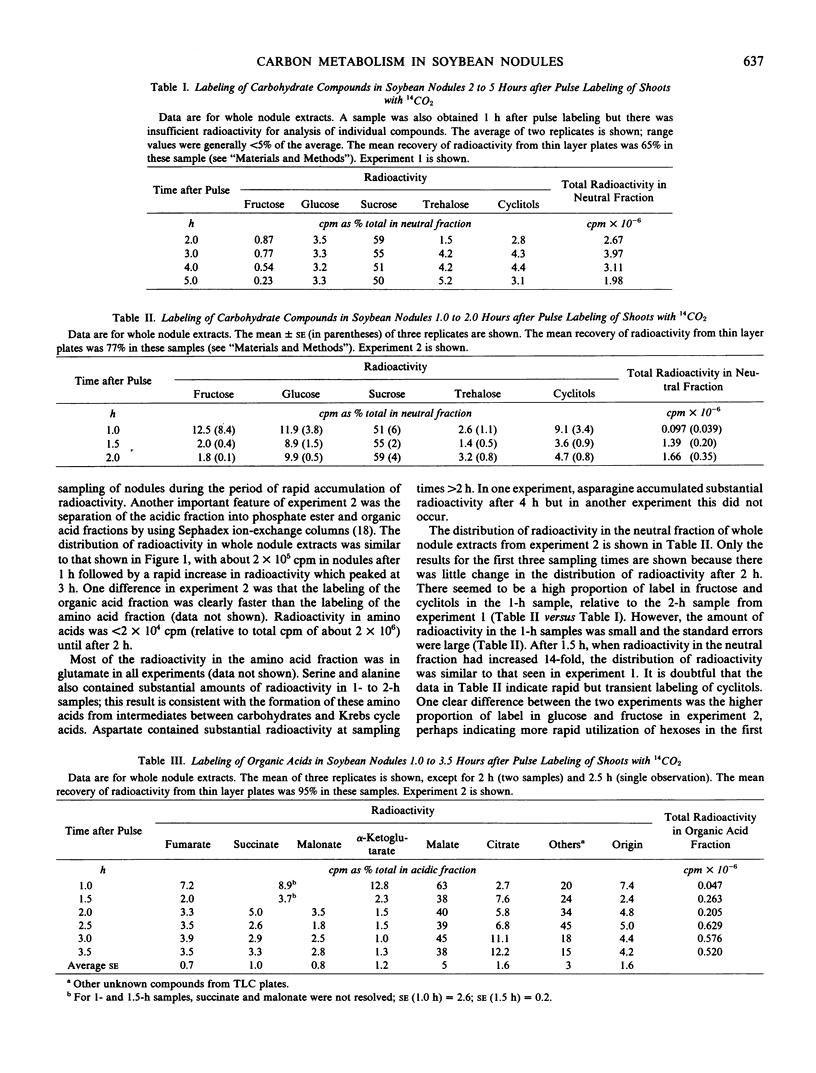
The metabolism of translocated photosynthate by soybean (Glycine max L. Merr.) nodules was investigated by 14CO2-labeling studies and analysis of nodule enzymes. Plants were exposed to 14CO2 for 30 minutes, followed by 12CO2 for up to 5 hours. The largest amount of radioactivity in nodules was recovered in neutral sugars at all sampling times. The organic acid fraction of the cytosol was labeled rapidly. Although cyclitols and malonate were found in high concentrations in the nodules, they accumulated less than 10% of the radioactivity in the neutral and acidic fractions, respectively. Phosphate esters were found to contain very low levels of total label, which prohibited analysis of the radioactivity in individual compounds. The whole nodule-labeling patterns suggested the utilization of photosynthate for the generation of organic acids (principally malate) and amino acids (principally glutamate).
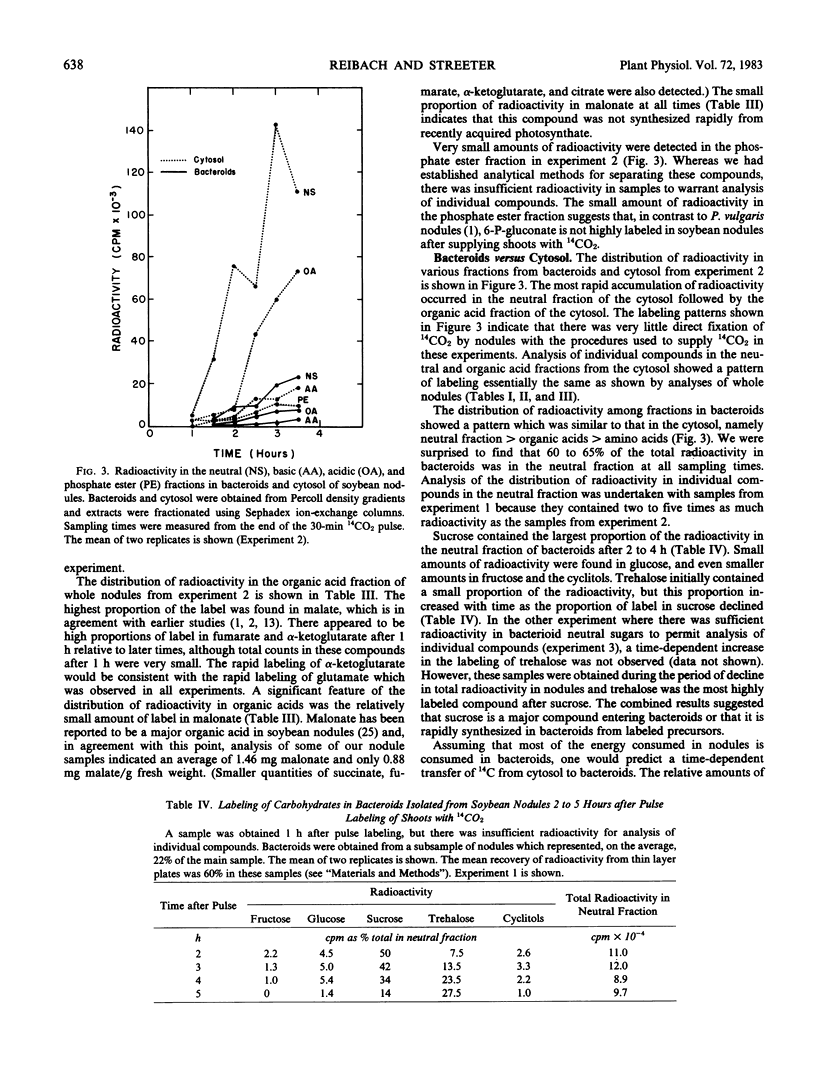
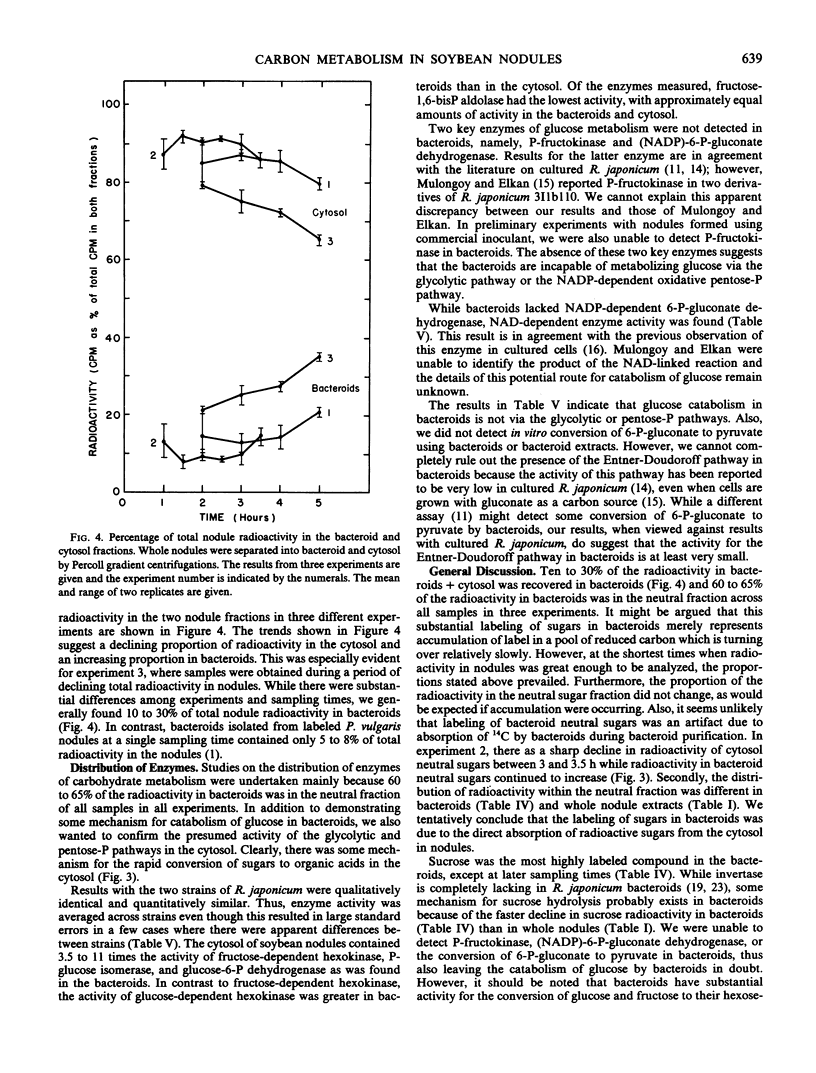
The radioactivity in bacteroids as a percentage of total nodule label increased slightly with time, while the percentage in the cytosol fraction declined. The labeling patterns for the cytosol were essentially the same as whole nodule-labeling patterns, and they suggest a degradation of carbohydrates for the production of organic acids and amino acids. When it was found that most of the radioactivity in bacteroids was in sugars, the enzymes of glucose metabolism were surveyed. Bacteroids from nodules formed by Rhizobium japonicum strain 110 or strain 138 lacked activity for phosphofructokinase and NADP-dependent 6-phosphogluconate dehydrogenase, key enzymes of glycolysis and the oxidative pentose-phosphate pathways. Enzymes of the glycolytic and pentose phosphate pathways were found in the cytosol fraction.
In three experiments, bacteroids contained about 10 to 30% of the total radioactivity in nodules 2 to 5 hours after pulse-labeling of plants, and 60 to 65% of the radioactivity in bacteroids was in the neutral sugar fraction at all sampling times. This strongly suggests some absorption and metabolism of sugars by bacteroids in spite of the lack of key enzymes. Bacteroids did possess enzymes for the formation of hexose phosphates from glucose or fructose. Radioactivity in α,α-trehalose in bacteroids increased until, after 5 hours, trehalose was a major labeled compound in bacteroids. Thus, trehalose synthesis may be a major fate of sugars entering bacteroids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGERSEN F. J. The bacterial component of soybean root nodules; changes in respiratory activity, cell dry weight and nucleic acid content with increasing nodule age. J Gen Microbiol. 1958 Oct;19(2):312–323. doi: 10.1099/00221287-19-2-312. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Magee W. E., Burris R. H. Translocation of Photosynthetic Products to Soybean Nodules and Their Role in Nitrogen Fixation. Plant Physiol. 1958 Mar;33(2):118–124. doi: 10.1104/pp.33.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. Glucose catabolism in two derivatives of a Rhizobium japonicum strain differing in nitrogen-fixing efficiency. J Bacteriol. 1977 Jul;131(1):179–187. doi: 10.1128/jb.131.1.179-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulongoy K., Elkan G. H. The role of 6-phosphogluconate dehydrogenase in Rhizobium. Can J Microbiol. 1977 Sep;23(9):1293–1298. doi: 10.1139/m77-193. [DOI] [PubMed] [Google Scholar]
- Raushel F. M., Cleland W. W. Bovine liver fructokinase: purification and kinetic properties. Biochemistry. 1977 May 17;16(10):2169–2175. doi: 10.1021/bi00629a020. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980 Sep 1;107(1):44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Reibach P. H., Mask P. L., Streeter J. G. A rapid one-step method for the isolation of bacteroids from root nodules of soybean plants, utilizing self-generating Percoll gradients. Can J Microbiol. 1981 May;27(5):491–495. doi: 10.1139/m81-072. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrates in Soybean Nodules: II. DISTRIBUTION OF COMPOUNDS IN SEEDLINGS DURING THE ONSET OF NITROGEN FIXATION. Plant Physiol. 1980 Sep;66(3):471–476. doi: 10.1104/pp.66.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Organic Acid contents of soybean: age and source of nitrogen. Plant Physiol. 1981 Nov;68(5):989–991. doi: 10.1104/pp.68.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Separation and detection of organic acids on silica gel. Anal Biochem. 1965 Sep;12(3):571–578. doi: 10.1016/0003-2697(65)90224-1. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., ARONOFF S. Metabolism of soybean leaves. IV. Translocation from soybean leaves. Arch Biochem Biophys. 1952 Apr;36(2):383–398. doi: 10.1016/0003-9861(52)90424-4. [DOI] [PubMed] [Google Scholar]


