Abstract
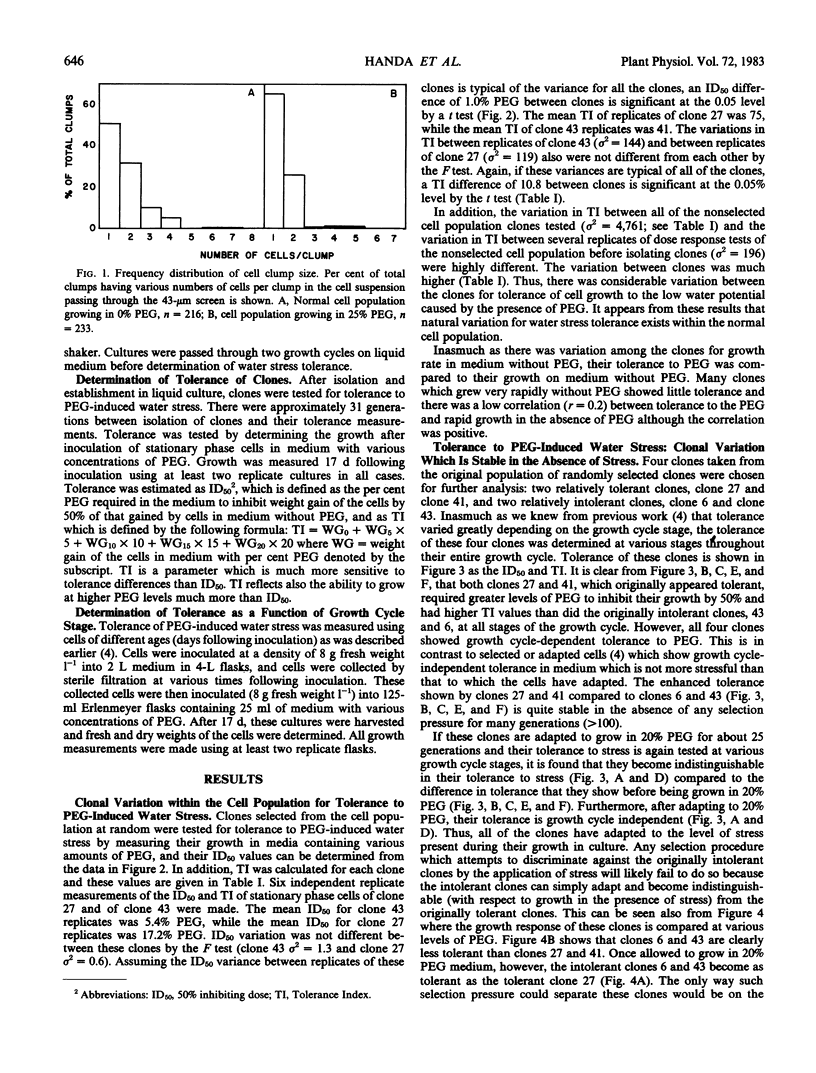
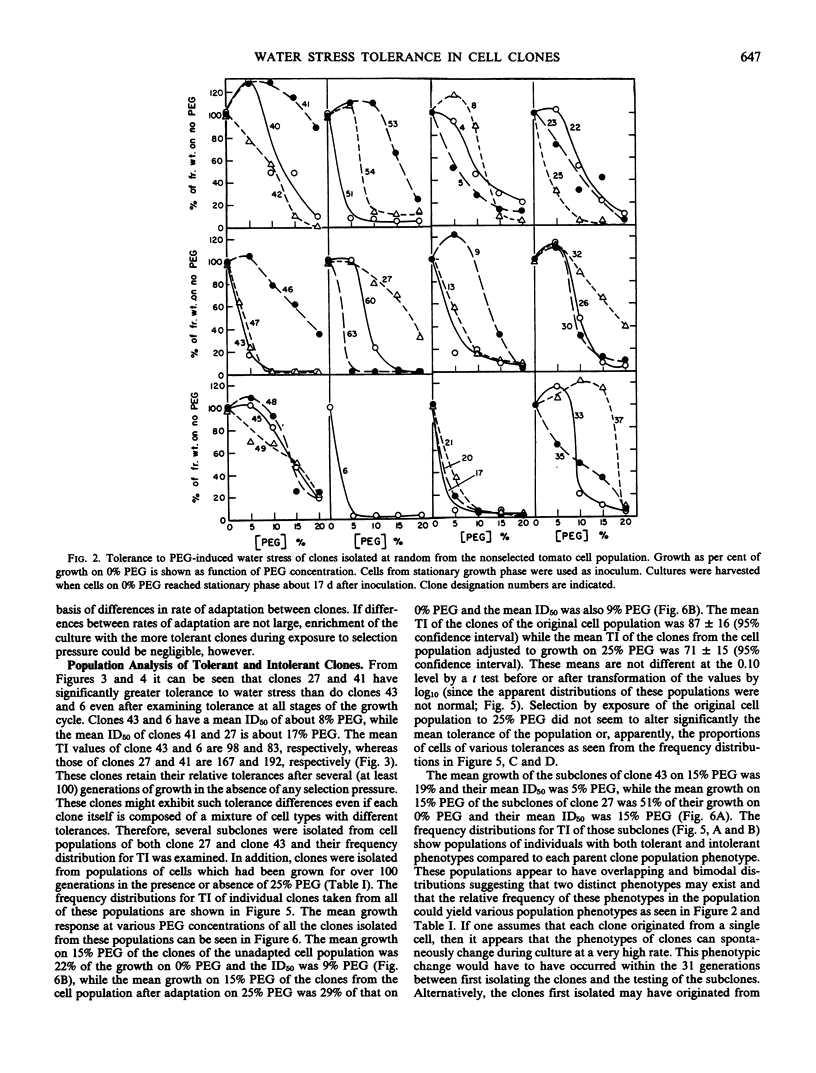
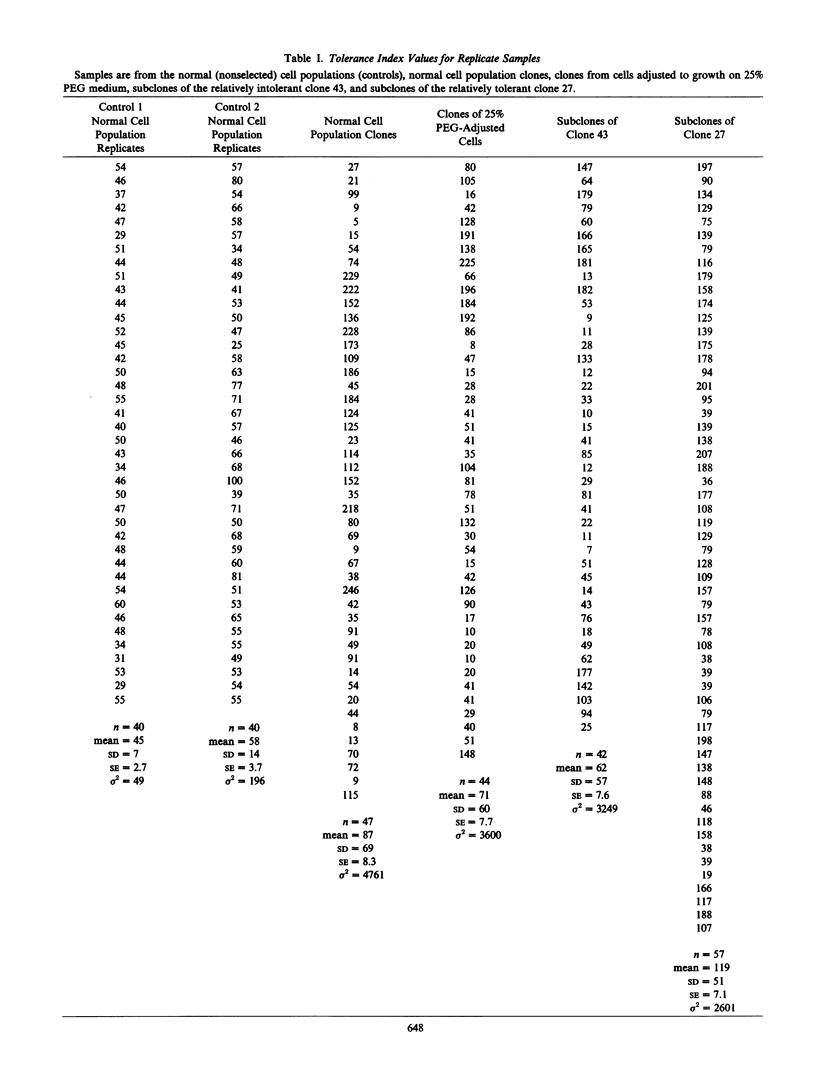
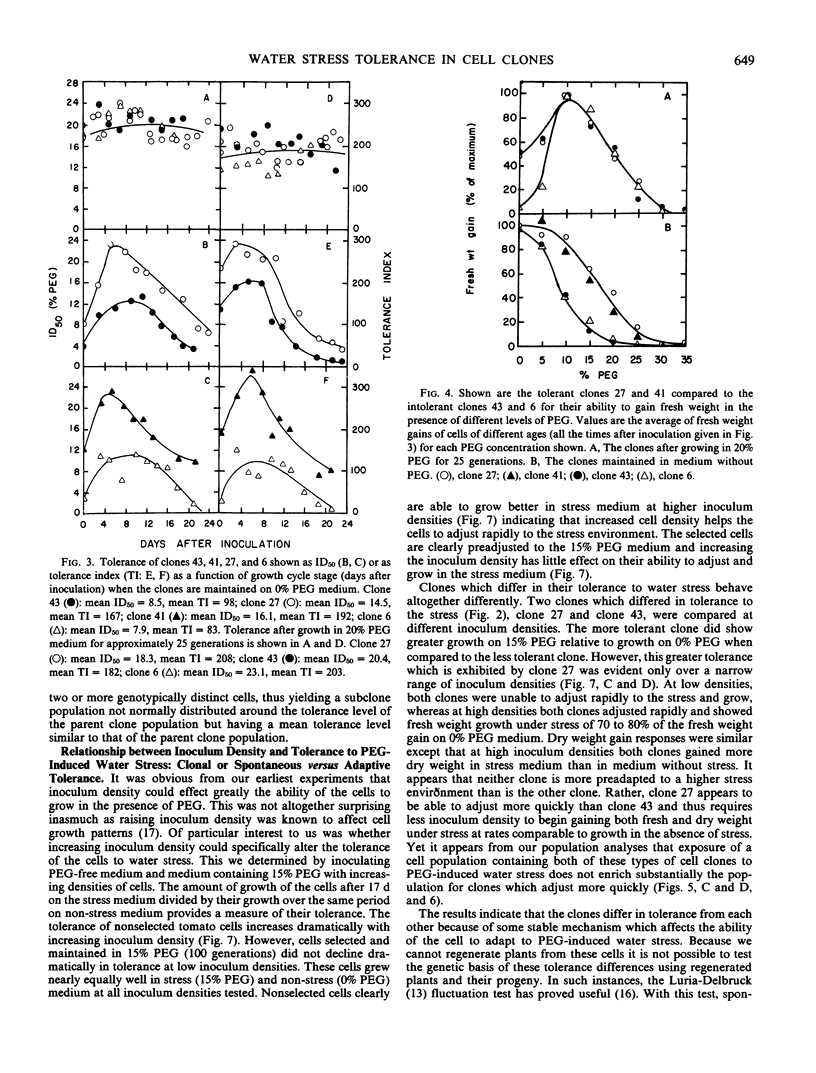
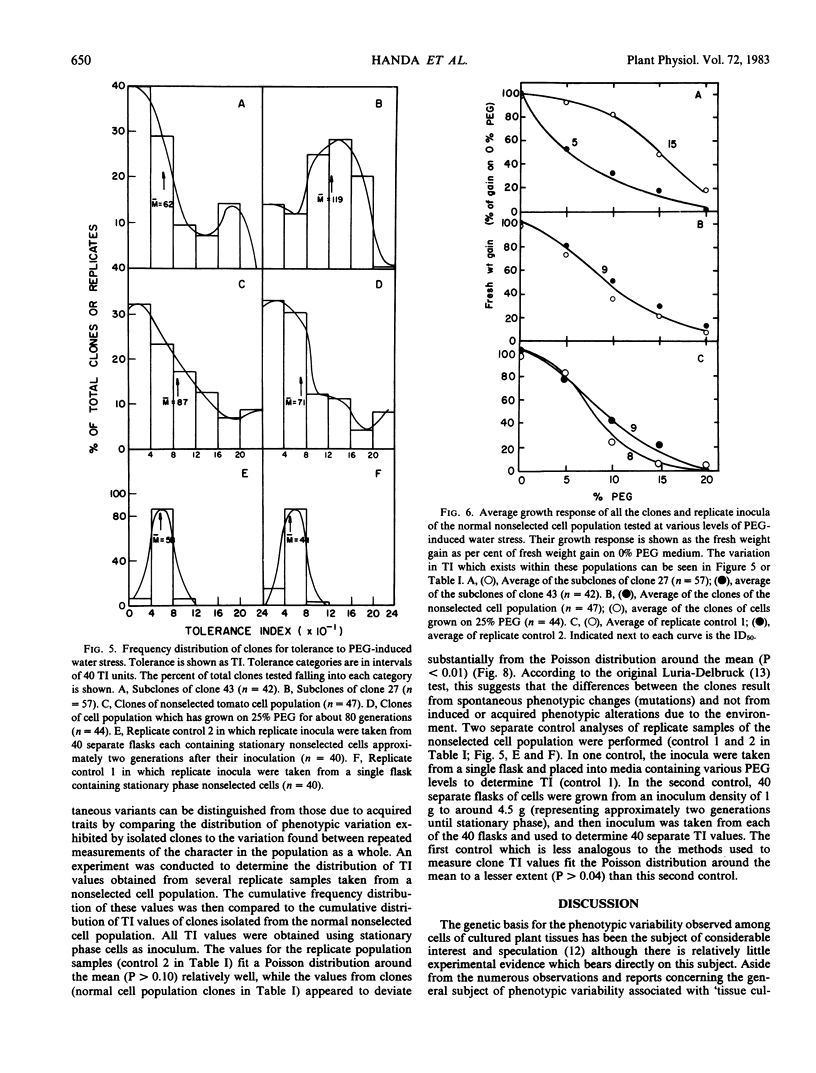
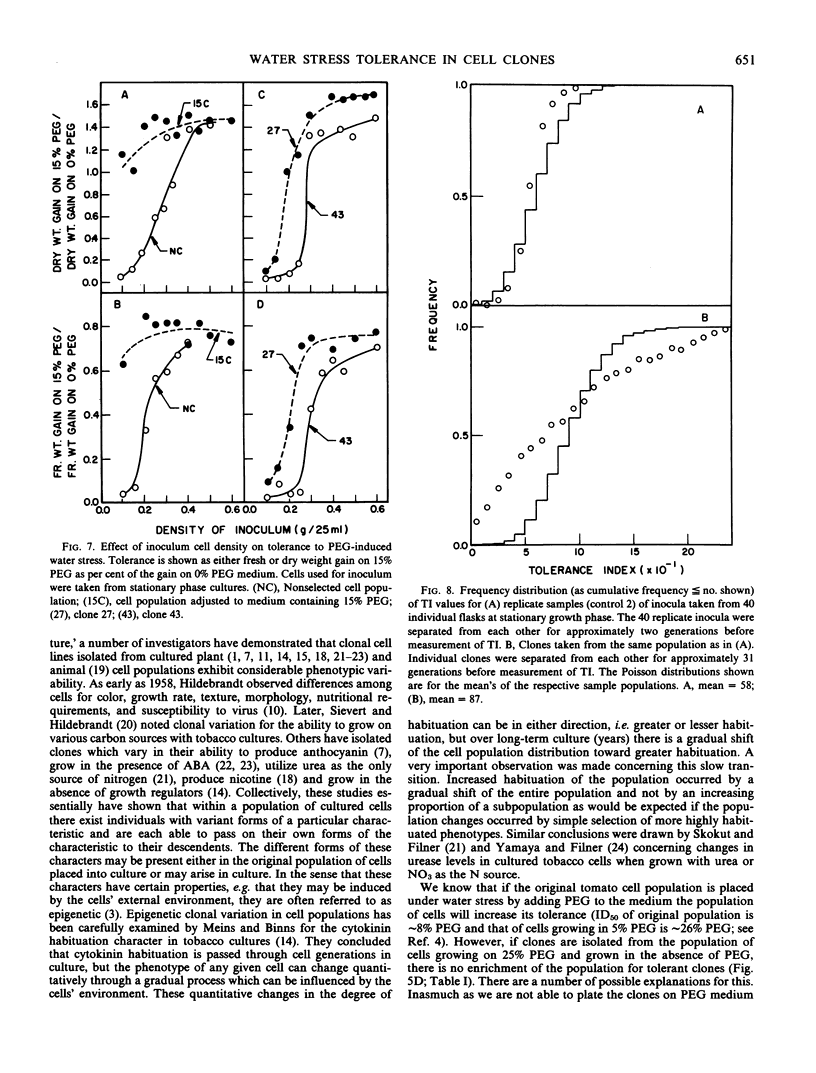
Cell clones were isolated from a population of cultured tomato (Lycopersicon esculentum Mill cv VFNT-cherry) cells and their tolerance to polyethylene glycol (PEG)-induced water stress was measured. Considerable variation for tolerance among the clones was found. Tolerance differences between clones appeared to be spontaneous and were different from tolerance differences between adapted and unadapted cells. Unlike adapted (selected by exposure to PEG) cells, cell clones retained their relative tolerance for many generations in the absence of selection pressure, and tolerance of both relatively tolerant and intolerant clones was very dependent on growth cycle stage and inoculum density. Analysis of subclones isolated from relatively tolerant and intolerant parent clones revealed that each parent clone gives rise to progeny with tolerances near the mean tolerance of both parents. However, progeny populations of both tolerant and intolerant parents are enriched with individuals with phenotypes nearer the mean response of their respective parent populations. When exposed to PEG, relatively tolerant and intolerant clones alike become adapted to the level of PEG to which they are exposed, and have the same phenotypic level of tolerance. Thus, selection by exposure to stress is unable to discriminate (on the basis of growth) between the innately tolerant and intolerant cell types within the population. This is indicated also by the fact that clones isolated from a population of cells adjusted to growth on 25% PEG do not show an enriched frequency of tolerant phenotypes when grown in the absence of PEG compared to the nonselected normal cell population which has never been adjusted to growth on PEG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMANN L. Growth and division of single cells of higher plants in vitro. J Gen Physiol. 1960 Mar;43:841–851. doi: 10.1085/jgp.43.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Handa A. K., Handa S., Hasegawa P. M. Growth and water relations of cultured tomato cells after adjustment to low external water potentials. Plant Physiol. 1982 Nov;70(5):1303–1309. doi: 10.1104/pp.70.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A. K., Bressan R. A., Handa S., Hasegawa P. M. Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol. 1982 Feb;69(2):514–521. doi: 10.1104/pp.69.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A. C. Stimulation or Inhibition of Virus of Infected and Insect-Gall Tissues and Single-Cell Clones. Proc Natl Acad Sci U S A. 1958 Apr;44(4):354–363. doi: 10.1073/pnas.44.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins F., Jr, Binns A. Epigenetic variation of cultured somatic cells: evidence for gradual changes in the requirement for factors promoting cell division. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2928–2932. doi: 10.1073/pnas.74.7.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe I. M., Cugnoli C. Isolation and characterization of a water-soluble photopigment from honeybee compound eye. Vision Res. 1980;20(2):97–102. doi: 10.1016/0042-6989(80)90150-9. [DOI] [PubMed] [Google Scholar]
- Peterson J. A. Analysis of discontinuous variation in albumin production by hepatoma cells at the cellular level. Somatic Cell Genet. 1979 Sep;5(5):641–651. doi: 10.1007/BF01542700. [DOI] [PubMed] [Google Scholar]
- SIEVERT R. C., HILDEBRANDT A. C. VARIATIONS WITHIN SINGLE CELL CLONES OF TOBACCO TISSUE CULTURES. Am J Bot. 1965 Aug;52:742–750. [PubMed] [Google Scholar]
- Skokut T. A., Filner P. Slow adaptive changes in urease levels of tobacco cells cultured on urea and other nitrogen sources. Plant Physiol. 1980 May;65(5):995–1003. doi: 10.1104/pp.65.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Filner P. Resistance to acetohydroxamate acquired by slow adaptive increases in urease in cultured tobacco cells. Plant Physiol. 1981 Jun;67(6):1133–1140. doi: 10.1104/pp.67.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]


