Abstract
Background and Objectives
High disease activity and frequent therapy failure in pediatric multiple sclerosis (MS) make prognostic biomarkers urgently needed. We investigated whether serum neurofilament light chain (sNfL) levels in treatment-naive pediatric patients with MS are associated with early disease severity and indicate treatment outcomes.
Methods
A retrospective cohort study of patients seen in the Göttingen Center for MS in Childhood and Adolescence, Germany. Inclusion criteria were MS diagnosis according to the McDonald criteria, MS onset <18 years, and available pretreatment serum sample. sNfL levels were analyzed using a single-molecule array assay. Associations with clinical and MRI evidence of disease severity at sampling were evaluated using the Spearman correlations and nonparametric tests for group comparisons. Correlations between pretreatment sNfL and annualized relapse and new T2 lesion rate on first-line therapy, and odd ratios for switch to high-efficacy therapy were assessed.
Results
A total of 178 patients (116 women [65%]) with a mean sampling age of 14.3 years were included in the study. Pretreatment sNfL levels were above the ≥90th percentile reported for healthy controls in 80% of patients (median 21.1 pg/mL) and correlated negatively with age, but no correlation was seen with sex, oligoclonal band status, or body mass index. High pretreatment sNfL levels correlated significantly with a high number of preceding relapses, a shorter first interattack interval, a high T2 lesion count, and recent gadolinium-enhancing lesions. Of interest, sNfL levels reflected more strongly MRI activity rather than clinical activity. Pretreatment sNfL levels also correlated significantly with the relapse rate and occurrence of new/enlarging T2 lesions while on first-line injectable therapy. Odds of future therapy escalation increased from 0.14 for sNfL below 7.5 pg/mL to 6.38 for sNfL above 15 pg/mL. In patients with a recent relapse, higher sNfL levels were associated with poorer recovery 3 months after attack.
Discussion
The results of this study have 3 important implications: First, pretreatment sNfL levels are a valuable biomarker for underlying disease activity in pediatric patients with MS. Second, pretreatment sNfL levels in pediatric patients with MS have a predictive value for the response to first-line therapy and the necessity of future therapy escalation. Third, high sNfL levels during a relapse are associated with poor recovery in this age group.
Introduction
Clinical onset in pediatric patients with multiple sclerosis (MS) is possibly closer to biological disease onset. This provides a unique opportunity for early therapeutic intervention, particularly because the initial disease course is almost exclusively relapsing-remitting without evidence of chronic progression.1,2 A challenging aspect of pediatric MS, however, is that the disease is characterized by greater inflammatory activity as evidenced by more relapses and faster accumulation of lesions compared with adult MS.3-6 Extensive axonal damage and axonal loss accompany the inflammation, aligning with early findings in children of cognitive impairment and brain atrophy.7-12 Although disability progression seems favorably slower in pediatric patients, any suggestion of a more benign course is dispelled by the fact that these patients are considerably younger when disability milestones are reached.1,13,14 Thus, prompt and effective therapy is imperative, yet high rates of breakthrough disease on the first-line disease-modifying therapies (DMTs) and protracted switch times to higher-efficacy therapies remain problematic issues.15-20 A sensitive, reliable way to assess disease severity and predict future disease progression would be an immense help to optimizing early treatment decisions. Emerging as a biomarker with great promise in this respect is neurofilament light chain (NfL).21-27
NfL is a neuron-specific, cytoskeletal protein abundantly found within myelinated axons in the peripheral nervous system and CNS.28 It is detectable in CSF and blood of healthy people, with levels increasing throughout the aging process.22,25,28 After any neuronal insult, whether traumatic, ischemic, inflammatory, or neurodegenerative, NfL levels rise significantly, making it a suitable marker of neuroaxonal damage but an unspecific indicator of etiology.29-33 Adult MS studies indicate the clinical application of NfL in facilitating early MS diagnosis, assessing disease activity, monitoring treatment response, and prognosticating future disease activity and disability progression.21-27
Few studies have investigated serum NfL (sNfL) levels in pediatric MS.34-36 Findings to date indicate that sNfL levels correlate strongly with CSF levels and are higher in children with MS compared with healthy children.35,36 Furthermore, sNfL levels have been associated with disease activity on MRI and shown to have potential application for monitoring treatment response.34-37
The main focus of this study was to investigate the clinical value of sNfL in the assessment of treatment-naive pediatric patients with MS. We investigated whether sNfL is a reliable indicator of early disease severity and can predict ongoing disease progression under first-line treatment and the likelihood of switching to higher-efficacy agents.
Methods
In this retrospective, single-center cohort study, cases in whom serum was collected before initiation of DMT were selected from the MS database of the Göttingen Centre for MS in Childhood and Adolescence—a tertiary referral center in Germany for pediatric MS. To allow for sufficient follow-up time after treatment initiation, no case in whom the serum sampling took place later than 2017 was included. Serum samples were not necessarily collected at the time of first clinical attack because some study cases were referred to Göttingen after previous clinical events. Additional study inclusion criteria were a confirmed diagnosis of relapsing-remitting MS fulfilling the revised McDonald MS diagnostic criteria (2005, 2010, or 2017), a clinical onset before 18 years, and no known history of traumatic injury or other CNS disease.38-40 Although no known myelin oligodendrocyte glycoprotein antibody (MOG-Ab)-positive patient was included in the study, routine screening for MOG-Abs was first initiated in Göttingen in 2013. Patients diagnosed before 2013 with atypical clinical and paraclinical findings for MS, particularly children with a first clinical event younger than 10 years, were also screened for MOG-Abs if still available to follow-up.
Serum samples were stored at −20°C and analyzed for NfL between January 2018 and August 2019 using the Quanterix Simoa NF-Light assay Advantage Kit (Lexington, MA) adhering to the manufacturer's instruction protocol. All samples were analyzed in duplicate. Testing was performed blinded to patient clinical/paraclinical data and outcome measures.
To assess disease activity at the time of sampling, clinical data were obtained from database and medical chart review. Data included sex, age, body mass index (BMI) within 6 months of serum collection, oligoclonal band (OCB) status in CSF before serum collection, the number of relapses before sampling and first interattack interval. Clinical relapses were defined as new or worsening neurologic symptoms lasting 24 hours or longer, occurring at least 30 days from a previous attack, and not accompanied by fever or evidence of infection. A recent relapse was defined as a relapse up to 90 days before sNfL sampling. In patients with a recent relapse, attack symptoms (grouped as visual, motor, sensory, brainstem, cerebellar, cerebral, and spinal) and recovery status (complete/incomplete) 3 months after attack were used to assess attack severity. An incomplete recovery was defined as an Expanded Disability Status Scale (EDSS) score ≥1.41 Recovery status was not included if another attack in ≤3 months had occurred.
For associations with a subclinical activity, manual lesion counts were obtained from brain and, if available, spinal MRIs performed before and after DMT initiation. Pretreatment T2 lesion counts were ≤6 months before NfL sampling, and gadolinium-enhancing (Gd+) lesion counts ≤30 days before sampling. Patients initiated on DMT and followed-up in Göttingen received 6-monthly MRI investigations. A volumetric analysis of lesion volume was not performed. In single cases where a substantial enlargement of a previous lesion was apparent, findings were included as evidence of ongoing activity. MRIs were acquired on a 1.5 or 3.0 Tesla scanner. Analyzed were T2-weighted, T2-fluid-attenuated inversion recovery, and T1-weighted sequences following contrast application. MRI assessment was performed blinded to sNfL findings by B.H and H.H.
Owing to the long sampling period in this study, treatment policies in Göttingen have undergone several changes. Until 2006, almost all patients were treated with interferon-beta (IFN-β) or glatiramer acetate (GA). From 2006, natalizumab was introduced for patients with very highly active disease, and from 2011, fingolimod. However, because both DMTs were not approved for use in this age group, most study patients initially received treatment with IFN-β or GA. Patients initiated on IFN-β or GA ≤3 months after sNfL-sampling who had ≥6 months follow-up were analyzed for ongoing disease activity under treatment (new relapses, new/enlarging T2 lesions at 6-monthly MRI follow-up). For each patient, the annualized rates were calculated for the available treatment follow-up; patients with annualized rates of zero had at least 12 months treatment follow-up. Because most patients were treated with IFN-β, IFN-β and GA were analyzed together.
Switch to high-efficacy treatment (fingolimod, natalizumab, rituximab, or alemtuzumab) during the follow-up duration was assessed only in cases with a clinical onset ≥2009 because of changing therapeutic practices.
The EDSS scores by Kurtzke, performed and documented by trained pediatric neurologists, at 2 years and the last follow-up were also collected to assess disability progression.41
Statistical Analysis
Descriptive statistics report counts and percentages for categorical variables, median, and interquartile range (IQR) for variables with skewed distribution, otherwise mean and SD. For correlation analysis and group comparisons involving sNfL, we used nonparametric techniques. The Spearman rank correlation was used to analyze continuous variables. Between-group comparisons were performed using either the Mann-Whitney U Test or Kruskal-Wallis test adjusted for multiple paired comparisons using a Bonferroni correction. The alpha level was set at 0.05, and all tests were 2-sided.
Two standard multiple linear regression models were used to analyze predictors of log-transformed sNfL. In model 1, the predictors were age, sex, presampling disease duration (log-transformed), recent relapse ≤90 days before sampling, >1 relapse in 12 months preceding sampling, recent presence of Gd+ lesions, and the number of T2 lesions (log-transformed). In model 2, the added influence of attack severity was analyzed by also including an incomplete recovery 3 months after a recent presampling attack as a predictor.
Logistic regression was used to assess the impact of 3 pretreatment predictors (T2 lesions >9, Gd+ lesions >1, and pretreatment sNfL >12.5 pg/mL, approximate 99th-percentile in healthy children) on the odds of developing a relapse or ≥2 new/enlarging T2 lesions in the first 12 months of IFN-β or GA treatment.36 The odds of a future switch from first- to second-line therapy were also analyzed using sNfL cut-offs of 7.5 (80th–90th percentile in healthy children), 10 (>90th percentile in healthy children), 12.5 (approximate 99th-percentile in healthy children), and 15 pg/mL, controlling for disease duration in a subgroup of patients with clinical onset ≥2009.36 For escalated patients, only disease duration up to the time point of escalation was used.
Statistical analyses were performed using IBM SPSS (version 28; SPSS Inc., Chicago, IL). Graphs were created with GraphPad Prism software version 9.3.1. Missing data were not imputed.
Standard Protocol Approvals, Registration, and Patient Consents
The study was approved by the Ethics Committee of the University Medical Center Göttingen, Georg August University Göttingen, Germany (number: 21/12/03). Written informed consent was obtained from all study participants and their legal guardians, for the collection and use of their clinical data and serum.
Data Availability
Anonymized data related to this article will be made available by request from any qualified investigator for the purpose of replicating results. Persons interested in obtaining data should contact Brenda Marie Huppke (Brenda.Huppke@med.uni-jena.de).
Results
Characteristics of the Cohort
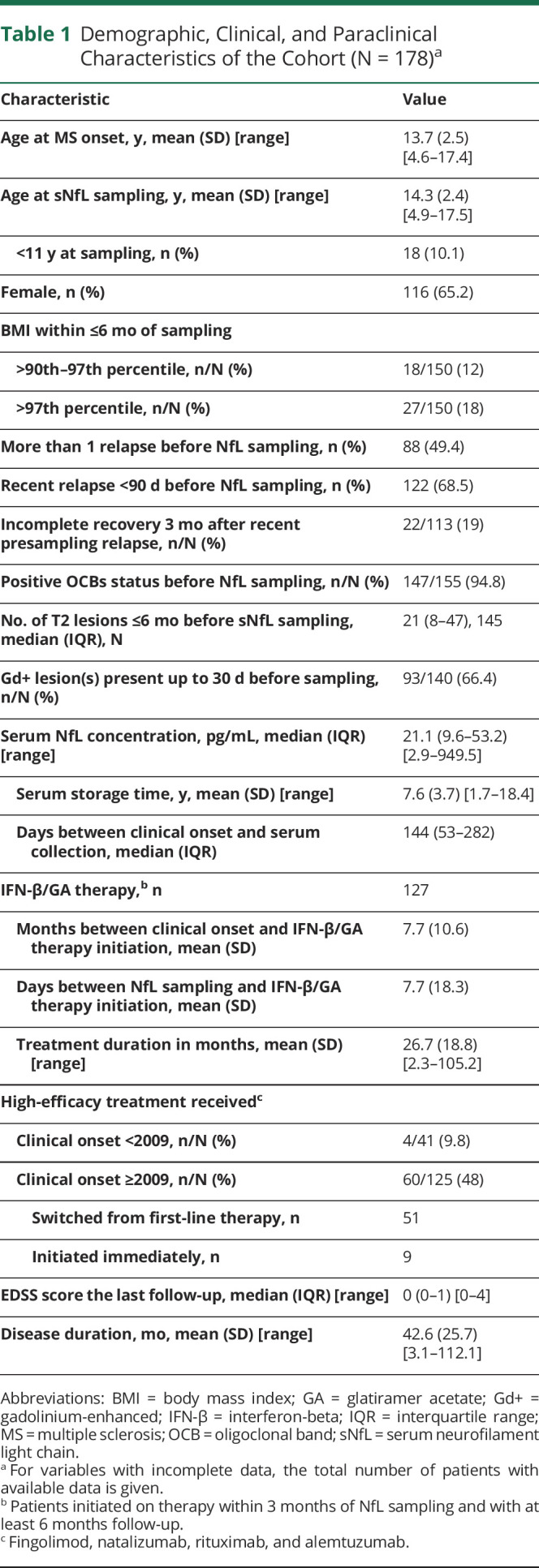
A total of 178 children and adolescents (116 women, 65%) with MS onset between 1998 and 2017 were included; 90% had an onset after 2005. The mean age at onset was 13.7 (SD 2.5, range 4.6–17.4) years, and at serum sampling, 14.3 (SD 2.4, range 4.9–17.5) years. Disability was low (median EDSS 0; IQR 0–1) after a mean (SD) follow-up of 42.6 months (25.7). Cohort characteristics are shown in Table 1.
Table 1.
Demographic, Clinical, and Paraclinical Characteristics of the Cohort (N = 178)a
| Characteristic | Value |
| Age at MS onset, y, mean (SD) [range] | 13.7 (2.5) [4.6–17.4] |
| Age at sNfL sampling, y, mean (SD) [range] | 14.3 (2.4) [4.9–17.5] |
| <11 y at sampling, n (%) | 18 (10.1) |
| Female, n (%) | 116 (65.2) |
| BMI within ≤6 mo of sampling | |
| >90th–97th percentile, n/N (%) | 18/150 (12) |
| >97th percentile, n/N (%) | 27/150 (18) |
| More than 1 relapse before NfL sampling, n (%) | 88 (49.4) |
| Recent relapse <90 d before NfL sampling, n (%) | 122 (68.5) |
| Incomplete recovery 3 mo after recent presampling relapse, n/N (%) | 22/113 (19) |
| Positive OCBs status before NfL sampling, n/N (%) | 147/155 (94.8) |
| No. of T2 lesions ≤6 mo before sNfL sampling, median (IQR), N | 21 (8–47), 145 |
| Gd+ lesion(s) present up to 30 d before sampling, n/N (%) | 93/140 (66.4) |
| Serum NfL concentration, pg/mL, median (IQR) [range] | 21.1 (9.6–53.2) [2.9–949.5] |
| Serum storage time, y, mean (SD) [range] | 7.6 (3.7) [1.7–18.4] |
| Days between clinical onset and serum collection, median (IQR) | 144 (53–282) |
| IFN-β/GA therapy,b n | 127 |
| Months between clinical onset and IFN-β/GA therapy initiation, mean (SD) | 7.7 (10.6) |
| Days between NfL sampling and IFN-β/GA therapy initiation, mean (SD) | 7.7 (18.3) |
| Treatment duration in months, mean (SD) [range] | 26.7 (18.8) [2.3–105.2] |
| High-efficacy treatment receivedc | |
| Clinical onset <2009, n/N (%) | 4/41 (9.8) |
| Clinical onset ≥2009, n/N (%) | 60/125 (48) |
| Switched from first-line therapy, n | 51 |
| Initiated immediately, n | 9 |
| EDSS score the last follow-up, median (IQR) [range] | 0 (0–1) [0–4] |
| Disease duration, mo, mean (SD) [range] | 42.6 (25.7) [3.1–112.1] |
Abbreviations: BMI = body mass index; GA = glatiramer acetate; Gd+ = gadolinium-enhanced; IFN-β = interferon-beta; IQR = interquartile range; MS = multiple sclerosis; OCB = oligoclonal band; sNfL = serum neurofilament light chain.
For variables with incomplete data, the total number of patients with available data is given.
Patients initiated on therapy within 3 months of NfL sampling and with at least 6 months follow-up.
Fingolimod, natalizumab, rituximab, and alemtuzumab.
sNfL Concentrations in Treatment-Naive Patients
Median sNfL concentration was 21.1 pg/mL (IQR 9.6–53.2, range 2.9–949.5) (Table 1); most (n = 143, 80%) had a level ≥90th percentile reported for healthy controls (7.7 pg/mL) (Table 1).36 Levels exceeding 100 pg/mL were uncommon (11%, n = 19). Only 13 patients (7.3%) had levels equivalent to the reported median for similarly aged healthy controls (4.6–5.1).36 Median time between clinical onset and serum collection was 144 days (IQR 53–282) and from most recent preceding relapse 45 days (IQR 21–112) (Table 1). No correlation was shown between sNfL and presampling disease duration (Spearman r = −0.02, p = 0.770) or serum storage time (Spearman r = 0.12, p = 0.101).
Associations Between sNfL and Age, Sex, BMI, OCB Status, and Recent Disease Activity
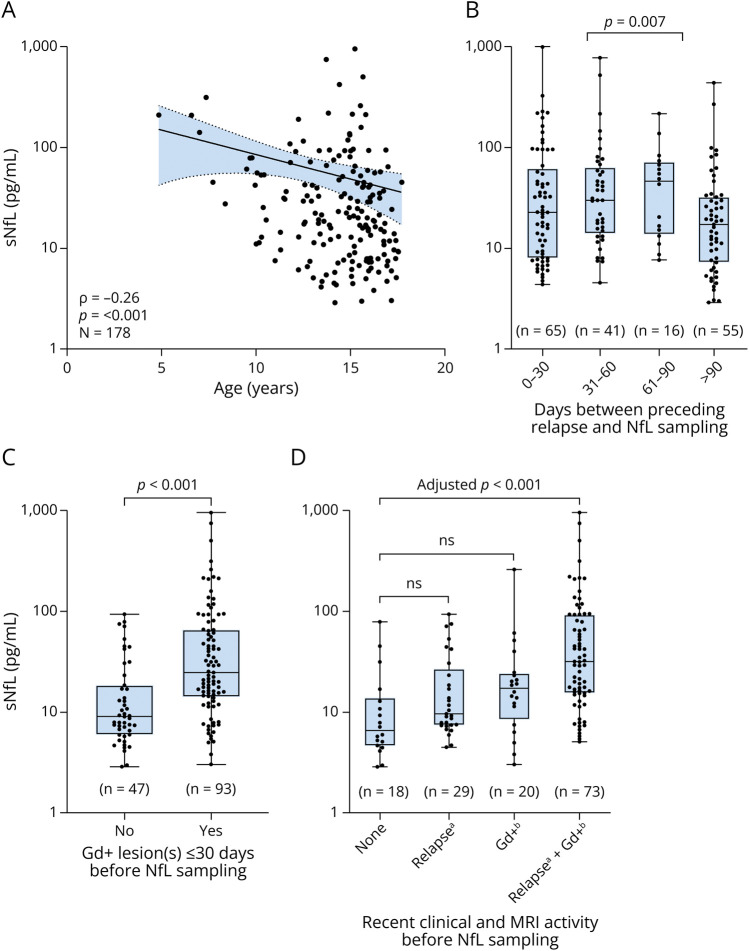
sNfL levels were negatively correlated with age (Spearman r = −0.26, p < 0.001) (Figure 1), even after controlling for BMI and presampling disease duration (r = −0.28, p < 0.001, n = 150). Levels in children younger than 11 years at sampling (n = 18) were significantly higher than in older patients (median [IQR] 53.4 [25.4–78.7] vs 19.0 [8.6–44.7], p = 0.004, respectively). sNfL levels were not associated with sex (p = 0.859), BMI (Spearman r = −0.12, p = 0.141, n = 150), BMI z-score (Spearman r = −0.03, p = 0.731), or OCB status (p = 0.852). Approximately two-thirds had experienced an attack up to 90 days before sampling (122/177, 69%), which was associated with higher sNfL levels (median [IQR] 27.1 [11.2–64.5] vs 16.9 [7.1–31.4], p = 0.007) (Figure 1). Recent Gd+ lesions (≤30 days before sampling), found in two-thirds of cases (93/140, 66%), were also associated with higher sNfL levels (median [IQR] 24.7 [14.4–65.4] vs 9.0 [6.0–18.5], p < 0.001) (Figure 1). Patients without clinical and MRI evidence of recent activity displayed the lowest sNfL values (median 6.5, IQR 4.7–12.9, n = 18), whereas those with combined clinical and MRI activity had the highest levels (median 31.8, IQR 15.5–92.3, adj. p < 0.001, n = 73) (Figure 1). Although the presence of Gd+ lesions was shown a significant unique predictor of log(sNfL) (β = 0.238, 95% CIs 0.07–0.40) in multivariable regression analyses, a recent relapse was not (Table 2).
Figure 1. Influence of Age and Recent Disease Activity on sNfL Levels in Treatment-Naive Pediatric Patients With MS.
A positive correlation between age at sampling and sNfL is shown in graph (A). Line indicates linear fit to the observed data and 95% confidence bands. Positive associations between sNfL and recent clinical and MRI activity before sampling are shown as boxplots in graph (B), (C), and (D). Boxes depict median and interquartile range with whiskers extending to minimum and maximum values. Gd+ = gadolinium-enhanced; MS = multiple sclerosis; sNfL = serum neurofilament light chain.
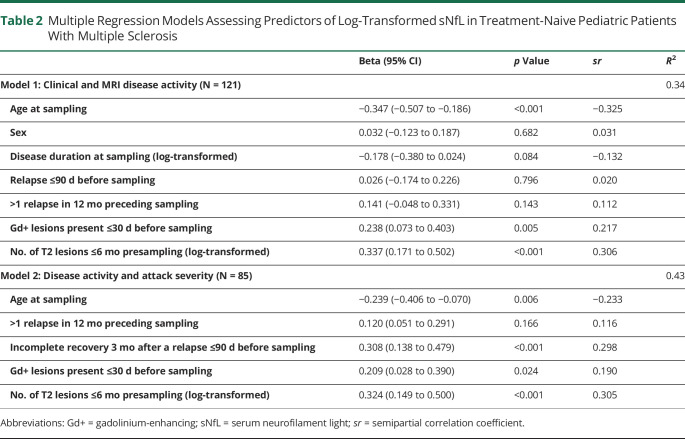
Table 2.
Multiple Regression Models Assessing Predictors of Log-Transformed sNfL in Treatment-Naive Pediatric Patients With Multiple Sclerosis
| Beta (95% CI) | p Value | sr | R 2 | |
| Model 1: Clinical and MRI disease activity (N = 121) | 0.34 | |||
| Age at sampling | −0.347 (−0.507 to −0.186) | <0.001 | −0.325 | |
| Sex | 0.032 (−0.123 to 0.187) | 0.682 | 0.031 | |
| Disease duration at sampling (log-transformed) | −0.178 (−0.380 to 0.024) | 0.084 | −0.132 | |
| Relapse ≤90 d before sampling | 0.026 (−0.174 to 0.226) | 0.796 | 0.020 | |
| >1 relapse in 12 mo preceding sampling | 0.141 (−0.048 to 0.331) | 0.143 | 0.112 | |
| Gd+ lesions present ≤30 d before sampling | 0.238 (0.073 to 0.403) | 0.005 | 0.217 | |
| No. of T2 lesions ≤6 mo presampling (log-transformed) | 0.337 (0.171 to 0.502) | <0.001 | 0.306 | |
| Model 2: Disease activity and attack severity (N = 85) | 0.43 | |||
| Age at sampling | −0.239 (−0.406 to −0.070) | 0.006 | −0.233 | |
| >1 relapse in 12 mo preceding sampling | 0.120 (0.051 to 0.291) | 0.166 | 0.116 | |
| Incomplete recovery 3 mo after a relapse ≤90 d before sampling | 0.308 (0.138 to 0.479) | <0.001 | 0.298 | |
| Gd+ lesions present ≤30 d before sampling | 0.209 (0.028 to 0.390) | 0.024 | 0.190 | |
| No. of T2 lesions ≤6 mo presampling (log-transformed) | 0.324 (0.149 to 0.500) | <0.001 | 0.305 |
Abbreviations: Gd+ = gadolinium-enhancing; sNfL = serum neurofilament light; sr = semipartial correlation coefficient.
Associations Between sNfL and Pretreatment Disease Severity
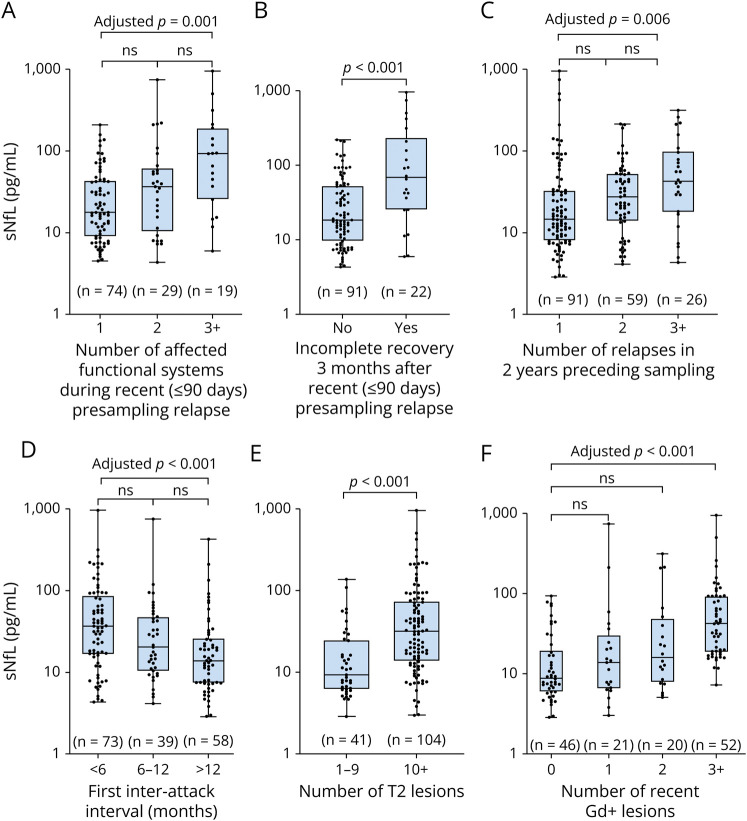
Nearly half the cohort (88/178, 49%) had experienced >1 attack before sampling (58 patients 2 relapses, 24 patients 3 relapses, 5 patients 4 relapses, and 1 patient 5 relapses). Higher sNfL levels were associated with a greater number of relapses in the previous 2 years (1 vs 2 vs ≥ 3, median [IQR] 14.6 [8.0–33.1] vs 27.5 [13.8–53.2] vs 42.9 [18.2–96.2], p = 0.004), a shorter first interattack interval (Spearman r = −0.32, p < 0.001, n = 149), presampling T2 lesions (Spearman r = 0.41, p < 0.001, n = 145), and recent Gd+ lesions (Spearman r = 0.55, p < 0.001, n = 140) (Figure 2, eFigure 1, links.lww.com/WNL/D105). In patients with a recent relapse up to 90 days before sampling, significantly higher sNfL levels were associated with multifocal neurology during the attack (1 vs 2 vs ≥ 3 affected functional systems, median [IQR] 17.7 [9.0–44.3] vs 35.8 [11.3–57.4] vs 92.3 [25.4–190.6], p = 0.001) and an incomplete recovery 3 months after attack (complete vs incomplete, median [IQR] 18.5 [9.6–53.2] vs 68.5 [25.4–209.4], p < 0.001) (Figure 2). Nearly two-thirds of patients with persisting neurology (14/22, 64%) also had multifocal neurology during the attack. sNfL concentrations exceeded 100 pg/mL in 36% (8/22) of cases with poor attack recovery, including the 5 highest cases in the study.
Figure 2. Associations Between sNfL Levels Recent Attack Severity and Clinical and Subclinical Disease at Sampling in Treatment-Naive Pediatric Patients With MS.
In patients with a recent relapse (≤90 days) before serum sampling, higher sNfL levels were associated with (A) polyfocal symptoms during the attack and (B) incomplete recovery 3 months after the attack. Both clinical (C, D) and subclinical disease (E, F) in the untreated patients showed associations with sNfL. Boxplots depict median and interquartile range with whiskers extending to minimum and maximum values. MS = multiple sclerosis; sNfL = serum neurofilament light chain.
In a multivariable linear regression model assessing clinical and MRI predictors of log(sNfL) (n = 121), significant unique predictors were age (β = −0.35, 95% CI −0.51 to −0.19), the number of T2 lesions (β 0.34, 95% CI 0.17–0.50), and recent presence of Gd+ lesions (β = 0.24, 95% CI 0.07–0.40) but not a recent relapse ≤90 days before sampling, >1 relapse in the preceding 12 months, and presampling disease duration (Table 2). In a second model (n = 85) assessing the added influence of attack severity, recovery status 3 months after a recent relapse before NfL sampling was the second strongest predictor (β = 0.31, 95% CI 0.14–0.48) of log(sNfL) after T2 lesion count (β = 0.32, 95% CI 0.15–0.50) (Table 2). Model 1 explained 34% and model 2 as much as 43% of the variance in log(sNfL).
Pretreatment sNfL and Treatment Outcome
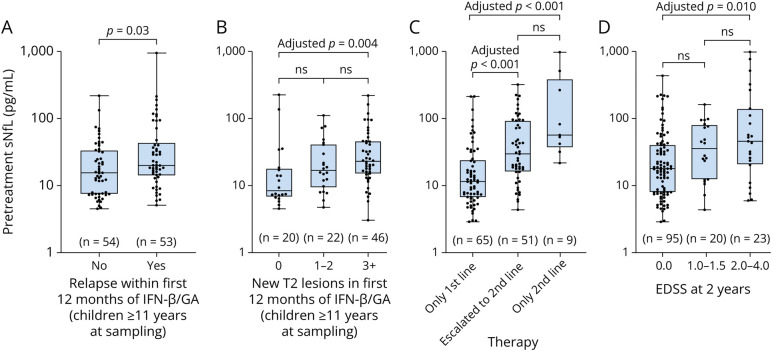
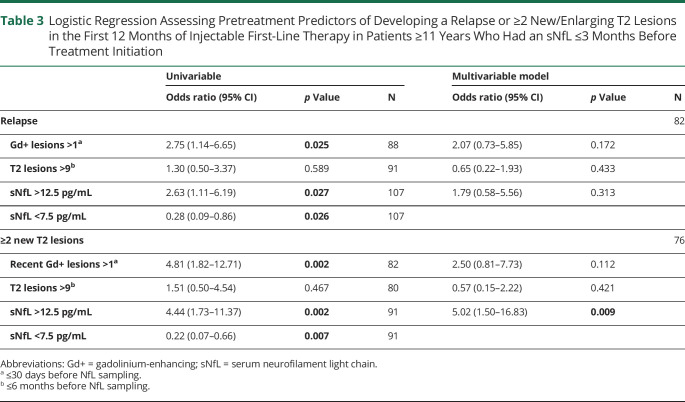
Altogether, 127 patients treated with IFN-β and/or GA for a mean (SD) treatment duration of 26.7 (18.8) months were included in the analyses (eFigure 2, links.lww.com/WNL/D106); mean (SD) time between treatment initiation and MS onset was 7.7 months (10.6) and from sNfL sampling only 7.7 days (18.3). Most patients were treated with IFN-β (n = 113). Only 8 patients received GA and 6 patients received IFN-β and GA, sequentially. Breakthrough disease was high among patients receiving an injectable first-line therapy. In children 11 years or older at treatment initiation, nearly 50% (53/107) had a relapse within the first 12 months of therapy and 77% (68/88) developed at least 1 new lesion. A positive correlation was found between sNfL levels before treatment and annualized relapse rate (Spearman r = 0.33, p < 0.001; n = 107) and annualized new/enlarging T2 lesion rate (Spearman r = 0.44; p < 0.001; n = 99) on IFN-β/GA therapy in patients ≥11 years at sampling, but not in younger children (eFigure 3, links.lww.com/WNL/D107). Considering only the first 12 months of first-line therapy in patients ≥11 years, sNfL values were also associated with relapse occurrence (no relapse vs relapse 15.5 [7.5–33.8] vs 20.0 [14.4–40.3], p = 0.030) and new T2 lesion development (0 vs 1–2 vs 3+ lesions 8.3 [6.8–17.5] vs 16.6 [9.6–40.1] vs 22.7 [14.9–45.0], p = 0.005) (Figure 3). An sNfL concentration >12.5 pg/mL (approximate 99th-percentile in healthy children) or recent Gd+ lesions >1 at serum sampling were related to more than 2 times greater odds of having a relapse (odds ratio [OR] 2.63, 95% CI 1.11–6.19 and 2.75, 95% CI 1.14–6.65, respectively) and more than 4-fold greater odds of developing ≥2 new/enlarging T2 lesions (OR 4.44, 95% CI 1.73–11.37 and 4.81, 95% CI 1.82–12.71, respectively) within the first 12 months of first-line therapy (Table 3). An sNfL concentration >12.5 pg/mL remained associated with lesion development (OR 5.02, 95% CI 1.50–16.53) as the only significant finding in multivariable analyses. T2 lesion counts >9 were not associated with greater odds of disease activity on the first-line therapy.
Figure 3. Associations Between Pretreatment sNfL Levels and Clinical and Subclinical Disease Activity on the First-Line Therapy, Need for Second-Line Therapy, and EDSS 2 Years After MS Onset.
Higher pretreatment sNfL levels up to 3 months before therapy initiation in patients ≥11 years were associated with a relapse (A) or new T2 lesion development (B) within the first 12 months of first-line therapy with IFN-β/GA. Patients with a clinical onset ≥2009 who had higher pretreatment sNfL levels were more likely to receive a high-efficacy therapy during the follow-up (C). Although most patients had no disability 2 years after onset, EDSS 2–4 was associated with higher pretreatment NfL concentrations (D). Boxplots depict median and interquartile range with whiskers extending to minimum and maximum values. EDSS = Expanded Disability Status Scale; GA = glatiramer acetate; IFN-β = interferon-beta; MS = multiple sclerosis; sNfL = serum neurofilament light chain.
Table 3.
Logistic Regression Assessing Pretreatment Predictors of Developing a Relapse or ≥2 New/Enlarging T2 Lesions in the First 12 Months of Injectable First-Line Therapy in Patients ≥11 Years Who Had an sNfL ≤3 Months Before Treatment Initiation
| Univariable | Multivariable model | |||||
| Odds ratio (95% CI) | p Value | N | Odds ratio (95% CI) | p Value | N | |
| Relapse | 82 | |||||
| Gd+ lesions >1a | 2.75 (1.14–6.65) | 0.025 | 88 | 2.07 (0.73–5.85) | 0.172 | |
| T2 lesions >9b | 1.30 (0.50–3.37) | 0.589 | 91 | 0.65 (0.22–1.93) | 0.433 | |
| sNfL >12.5 pg/mL | 2.63 (1.11–6.19) | 0.027 | 107 | 1.79 (0.58–5.56) | 0.313 | |
| sNfL <7.5 pg/mL | 0.28 (0.09–0.86) | 0.026 | 107 | |||
| ≥2 new T2 lesions | 76 | |||||
| Recent Gd+ lesions >1a | 4.81 (1.82–12.71) | 0.002 | 82 | 2.50 (0.81–7.73) | 0.112 | |
| T2 lesions >9b | 1.51 (0.50–4.54) | 0.467 | 80 | 0.57 (0.15–2.22) | 0.421 | |
| sNfL >12.5 pg/mL | 4.44 (1.73–11.37) | 0.002 | 91 | 5.02 (1.50–16.83) | 0.009 | |
| sNfL <7.5 pg/mL | 0.22 (0.07–0.66) | 0.007 | 91 | |||
Abbreviations: Gd+ = gadolinium-enhancing; sNfL = serum neurofilament light chain.
≤30 days before NfL sampling.
≤6 months before NfL sampling.
We investigated whether there was a subset of patients more likely to remain clinically and radiographically stable on low-efficacy injectables. Only 7 patients with sNfL levels equivalent to the reported median for similarly aged healthy controls (4.6–5.1, n = 13) had a treatment follow-up, and thus, we analyzed patients with a pretreatment sNfL <7.5 pg/mL (recently reported as 80th–90th percentile for healthy age equivalents).36 Odds of developing a relapse (OR 0.28, 95% CI 0.09–0.86) and/or ≥2 new/enlarging T2 lesions (OR 0.22, 95% CI 0.07–0.66) in the first year of low-efficacy injectable therapy (Table 3) and the odds of escalation to a second-line therapy (0.14, 95% CI 0.04–0.45) were considerably lower in this group of patients (Table 4).
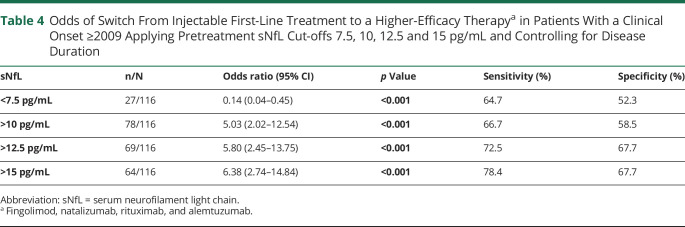
Table 4.
Odds of Switch From Injectable First-Line Treatment to a Higher-Efficacy Therapya in Patients With a Clinical Onset ≥2009 Applying Pretreatment sNfL Cut-offs 7.5, 10, 12.5 and 15 pg/mL and Controlling for Disease Duration
| sNfL | n/N | Odds ratio (95% CI) | p Value | Sensitivity (%) | Specificity (%) |
| <7.5 pg/mL | 27/116 | 0.14 (0.04–0.45) | <0.001 | 64.7 | 52.3 |
| >10 pg/mL | 78/116 | 5.03 (2.02–12.54) | <0.001 | 66.7 | 58.5 |
| >12.5 pg/mL | 69/116 | 5.80 (2.45–13.75) | <0.001 | 72.5 | 67.7 |
| >15 pg/mL | 64/116 | 6.38 (2.74–14.84) | <0.001 | 78.4 | 67.7 |
Abbreviation: sNfL = serum neurofilament light chain.
Fingolimod, natalizumab, rituximab, and alemtuzumab.
In patients with MS onset ≥2009 (n = 125, mean [SD] disease duration 40.7 months [23.0]), 51 (41%) were switched from first- to second-line therapy. Higher pretreatment sNfL levels were associated with future therapy switch (nonswitched vs switched, median [IQR] 11.3 [6.7–24.7] vs 29.3 [15.9–78.4], adj. p < 0.001), whereas highest levels were found in patients started directly on a high-efficacy therapy (median 55.7, IQR 41.4–259.6, adj. p < 0.001, n = 9); all of these patients had a pretreatment level >20 pg/mL (Figure 3). Controlling for disease duration, increasing pretreatment sNfL cut-off values (10, 12.5, 15 pg/mL) were associated with increasing odds of therapy switch to higher efficacy agents (Table 4).
Pretreatment sNfL and EDSS
Although the overall disability was low, pretreatment sNfL levels were associated with EDSS 2 years after MS onset (EDSS 0 vs 1–1.5 vs 2–4, median [IQR] 17.7 [7.9–40.1] vs 34.9 [12.1–79.4] vs 45.0 [20.0–137.0], p = 0.006) (Figure 3).
Discussion
Median sNfL concentration in treatment-naive patients in our cohort was 4-fold higher (21.1 pg/mL) than that reported in healthy children (4.7–5.1 pg/mL), supporting mounting evidence that the early inflammatory disease process in pediatric MS is associated with substantial neuroaxonal damage.7,8,34-36,42 The study's main findings suggest that sNfL levels before treatment initiation are an indicator of early disease severity and correlate with future clinical and subclinical activity under conventional first-line treatment.
Notably, age at sampling was found to influence sNfL levels with younger age associated with higher values. In healthy pediatric cohorts, sNfL levels have been shown highest in early infancy, declining abruptly up to approximately 4–6 years of age and then stabilizing until adulthood.36,42 Because all children except 1 in our cohort were older than 6 years, an age at which sNfL values stabilizes, the negative age association in pediatric MS may indicate an increased susceptibility to neuroaxonal damage in prepubertal children with MS, which is supported by brain biopsy findings.7 sNfL concentrations were not associated with sex, OCB status, disease duration at sampling, or serum storage time. Although higher BMIs have been associated with lower NfL blood levels in adult studies, possibly secondary to higher blood volumes, we found no significant influence of BMI.25,43
Most patients in our study had experienced recent disease activity before serum sampling, which is also likely in real-world settings before therapy initiation. A relapse up to 90 days and/or Gd+ lesions up to 1 month before testing were associated with significantly higher sNfL levels, consistent with recent findings.36,44 The presence of recent Gd+ lesions but not a recent relapse remained a significant predictor of sNfL levels in our multiple regression model, therefore it is possible that rising sNfL levels more strongly reflect MRI rather than clinical activity, matching at least 1 adult study.44 Elevated sNfL levels were found in more than a quarter of the cases without evidence of recent activity, suggesting that subacute disease activity and neuronal damage may be escaping routine detection methods in some patients.
Several of the study's associations indicate that sNfL levels reflect the degree of recent and past disease activity, suggesting a beneficial role of the biomarker in early disease severity assessment. Higher levels were associated with more relapses in the 2 years preceding sampling and higher numbers of T2 and Gd+ lesions, whereas the first interattack interval was inversely correlated. MRI findings match previous pediatric studies.34,36,37
Anticipating the risk of early disability development is another essential part of disease severity assessment, given the highly inflammatory nature and extensive axonal damage associated with pediatric MS.3,5,7 Because a severe attack has been shown to increase the risk of poor attack recovery which in turn has been identified as a predictor of disease progression, we investigated whether sNfL is an indicator of attack recovery.13,14 In patients with a relapse up to 90 days before sNfL sampling, higher sNfL concentrations were associated with an incomplete recovery 3 months after attack. Half of the patients with persisting neurology had an sNfL level exceeding 70 pg/mL related to the attack and more than a third above 100 pg/mL, indicating substantial axonal injury. Although this has not been described before in children, incomplete remission after optic neuritis has been associated with higher CSF NfL levels in adults.45 Our finding is relevant because it suggests that very high levels may signify slow attack recovery or even irreversible damage, which may be prevented by more aggressive therapy.
Most patients in this study had an onset of disease before the introduction of high-efficacy therapies and were therefore treated with IFN-β or GA. Although in Göttingen most pediatric patients are now started on higher-efficacy DMTs (fumarates, fingolimod, and natalizumab), it still common practice in many countries to begin with lower efficacy DMTs. Because refractory disease under first-line agents is frequent, the identification of patients likely to respond well or poorly to conventional first-line therapies remains a challenge for neurologists.15-20 Our results indicate that pretreatment sNfL assessment may help justify higher efficacy DMT choices in some patients. In our cohort, pretreatment sNfL levels in patients ≥11 years correlated with future relapses and new/enlarging T2 lesion development on IFN-β and GA therapy. A pretreatment sNfL >12.5 pg/mL (approximate 99th-percentile in a recent healthy children cohort) was associated with a 2-fold greater odds of a relapse and more than a 4-fold greater odds of developing ≥2 new T2 lesions in the first 12 months of the first-line therapy.36 A similar association with Gd+ lesions >1 at sampling was also found; however, because contrast enhancement is a transient finding that may escape detection, sNfL may serve as a potentially more reliable prognostic indicator in clinical practice. Pretreatment T2 lesion count >9 was not found prognostic of disease activity in the first year of treatment. Conversely, patients with sNfLs below 7.5 pg/mL showed considerably lower likelihood of disease progression within the first 12 months on the first-line therapy. This small group of pediatric patients with MS might remain candidates for the first-line therapy in the future. In pediatric MS, the prognostic potential of sNfL regarding injectable first-line treatment response has not been studied before. Nevertheless, higher sNfL concentrations in children and adolescents with a first demyelinating episode have been shown predictive of both a second attack and relapses over the next 2 years.34,35 Furthermore, a recent pediatric study reporting a post hoc analysis of patients receiving either a placebo or teriflunomide in the TERKIDS trial showed that higher baseline plasma NfL levels were associated with increased hazard of clinical and MRI activity during the observed double-blind period, supporting our findings.37 Several adult MS studies have also related higher baseline NfL levels to increased likelihood of future clinical and MRI activity on treatment, even in apparently stable patients showing no conventional evidence of disease activity.25-27,46-48 We found no association with future activity on the first-line therapy in younger children, despite higher levels before treatment initiation. Findings may have been limited by small sample size or, alternatively, indicate better response to first-line therapy in this age group. Findings in a recent Italian study of an association between age ≤12 years at therapy initiation on injectable first-line agents and fewer relapses and better long-term disability outcomes support the latter suggestion.19
Retrospectively, pretreatment sNfL levels reflected decisions for high-efficacy therapy, further supporting prognostic utility of pretreatment sNfL in pediatric MS. Highest levels were found in patients started directly on high-efficacy therapy, whereas increasing pretreatment sNfL cut-offs values (10, 12.5 and 15 pg/mL) were associated with increasing odds of future therapy escalation.
A strength of our study is the large pediatric MS sample size. Limitations, however, include its retrospective nature and potential case selection bias in favor of more severe disease as a tertiary referral center. Analyses were also limited by variable follow-up durations and incomplete availability of complete data sets, particularly MRI data and treatment follow-up data. We also cannot exclude the possibility that among patients lost to follow-up before the initiation of routine MOG-Ab screening in Göttingen in 2013, some cases with MOG-Ab disease may have been misdiagnosed as MS. Finally, because not all study patients had findings consistent with group level associations, further studies of such patients are needed to improve the application potential of sNfL as a predictive biomarker.
In conclusion, our results suggest the usefulness of pretreatment sNfL as a biomarker in improving early disease assessment in treatment-naive pediatric patients with MS. We uniquely show that sNfL may also be a useful biomarker for quantifying attack severity and signifying the risk of poor recovery. Furthermore, associations with disease activity on the first-line therapy and future need for higher efficacy agents indicate prognostic value for guiding initial therapy decisions.
Glossary
- BMI
body mass index
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- GA
glatiramer acetate
- Gd+
gadolinium-enhanced
- IFN-β
interferon-beta
- IQR
interquartile range
- MOG-Ab
myelin oligodendrocyte glycoprotein antibody
- MS
multiple sclerosis
- OCB
oligoclonal band
- OR
odds ratio
- sNfL
serum neurofilament light chain
Appendix. Authors
| Name | Location | Contribution |
| Brenda Huppke, MBChB | Department of Pediatric Neurology, University Hospital Jena, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis and interpretation of data |
| Marie-Christine Reinert, MD | Department of Pediatrics and Adolescent Medicine, Pediatric Neurology, University Medical Center Göttingen, Georg August University Göttingen, Germany | Major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Hannah Hummel-Abmeier, MD | Department of Pediatrics and Adolescent Medicine, Pediatric Neurology, University Medical Center Göttingen, Georg August University Göttingen, Germany | Major role in the acquisition of data and analysis or interpretation of data |
| Wiebke Stark, MD | Department of Pediatrics and Adolescent Medicine, Pediatric Neurology, University Medical Center Göttingen, Georg August University Göttingen, Germany | Major role in the acquisition of data and revision of the manuscript for content |
| Jutta Gärtner, MD | Department of Pediatrics and Adolescent Medicine, Pediatric Neurology, University Medical Center Göttingen, Georg August University Göttingen, Germany | Major role in the acquisition of data; study concept and design; interpretation of data; and revision of the manuscript for intellectual content |
| Peter Huppke, MD | Department of Neuropediatrics, University Hospital Jena, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
J. Gärtner, in the past 3 years, has received fees for lectures and consultancy fees from Bayer, Biogen, Merck, Novartis, and Sanofi. P. Huppke, in the past 3 years, has received speaker honoraria and consultancy fees from Biogen, Merck, Teva, and GW Pharma. All other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356(25):2603-2613. doi: 10.1056/nejmoa067597 [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt K, Weiss S, Rosenbauer J, Gartner J, von Kries R. Multiple sclerosis in children and adolescents: incidence and clinical picture: new insights from the nationwide German surveillance (2009-2011). Eur J Neurol. 2014;21(4):654-659. doi: 10.1111/ene.12371 [DOI] [PubMed] [Google Scholar]
- 3.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54-59. doi: 10.1001/archneurol.2008.505 [DOI] [PubMed] [Google Scholar]
- 4.Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord. 2014;3(2):186-193. doi: 10.1016/j.msard.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. 2009;66(8):967-971. doi: 10.1001/archneurol.2009.135 [DOI] [PubMed] [Google Scholar]
- 6.Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain. 2009;132(12):3392-3400. doi: 10.1093/brain/awp278 [DOI] [PubMed] [Google Scholar]
- 7.Pfeifenbring S, Bunyan RF, Metz I, et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol. 2015;77(4):655-667. doi: 10.1002/ana.24364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels F, Nobis K, Cooper G, et al. Childhood multiple sclerosis is associated with reduced brain volumes at first clinical presentation and brain growth failure. Mult Scler. 2019;25(7):927-936. doi: 10.1177/1352458519829698 [DOI] [PubMed] [Google Scholar]
- 9.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70(20):1891-1897. doi: 10.1212/01.wnl.0000312276.23177.fa [DOI] [PubMed] [Google Scholar]
- 10.Julian L, Serafin D, Charvet L, et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: results from a United States network. J Child Neurol. 2013;28(1):102-107. doi: 10.1177/0883073812464816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Till C, Ghassemi R, Aubert-Broche B, et al. MRI correlates of cognitive impairment in childhood-onset multiple sclerosis. Neuropsychology. 2011;25(3):319-332. doi: 10.1037/a0022051 [DOI] [PubMed] [Google Scholar]
- 12.Aubert-Broche B, Fonov V, Narayanan S, et al. Onset of multiple sclerosis before adulthood leads to failure of age-expected brain growth. Neurology. 2014;83(23):2140-2146. doi: 10.1212/wnl.0000000000001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fay AJ, Mowry EM, Strober J, Waubant E. Relapse severity and recovery in early pediatric multiple sclerosis. Mult Scler. 2012;18(7):1008-1012. doi: 10.1177/1352458511431725 [DOI] [PubMed] [Google Scholar]
- 14.Santoro JD, Waltz M, Aaen G, et al. Pediatric Multiple Sclerosis Severity Score in a large US cohort. Neurology. 2020;95(13):e1844-e1853. doi: 10.1212/wnl.0000000000010414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg B, Kolodny S, Wang M, Deshpande C. Utilization and treatment patterns of disease-modifying therapy in pediatric patients with multiple sclerosis in the United States. Int J MS Care. 2021;23(3):101-105. doi: 10.7224/1537-2073.2019-095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler. 2019;25(1):72-80. doi: 10.1177/1352458517732843 [DOI] [PubMed] [Google Scholar]
- 17.Erdal JL, Kopp TI, Blinkenberg M, Petersen T, Sorensen PS, Magyari M. Clinical characteristics and use of disease modifying therapy in the nationwide Danish cohort of paediatric onset multiple sclerosis. Mult Scler Relat Disord. 2020;37:101431. doi: 10.1016/j.msard.2019.101431 [DOI] [PubMed] [Google Scholar]
- 18.Krysko KM, Graves JS, Rensel M, et al. Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020;88(1):42-55. doi: 10.1002/ana.25737 [DOI] [PubMed] [Google Scholar]
- 19.Baroncini D, Zaffaroni M, Moiola L, et al. Long-term follow-up of pediatric MS patients starting treatment with injectable first-line agents: a multicentre, Italian, retrospective, observational study. Mult Scler. 2019;25(3):399-407. doi: 10.1177/1352458518754364 [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Mannan OA, Manchoon C, Rossor T, et al. Use of disease-modifying therapies in pediatric relapsing-remitting multiple sclerosis in the United Kingdom. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1008. doi: 10.1212/nxi.0000000000001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/wnl.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varhaug KN, Barro C, Bjornevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e422. doi: 10.1212/nxi.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine. 2020;56:102807. doi: 10.1016/j.ebiom.2020.102807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/s1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 26.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 27.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. doi: 10.1212/wnl.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gafson AR, Barthelemy NR, Bomont P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143(7):1975-1998. doi: 10.1093/brain/awaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67(5):2013-2018. doi: 10.1046/j.1471-4159.1996.67052013.x [DOI] [PubMed] [Google Scholar]
- 30.Lycke JN, Karlsson JE, Andersen O, Rosengren LE. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(3):402-404. doi: 10.1136/jnnp.64.3.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91(14):e1338-e1347. doi: 10.1212/wnl.0000000000006282 [DOI] [PubMed] [Google Scholar]
- 32.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol. 2016;3(8):623-636. doi: 10.1002/acn3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6(1):36791. doi: 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendel EM, Bertolini A, Kousoulos L, et al. Serum neurofilament light-chain levels in children with monophasic myelin oligodendrocyte glycoprotein-associated disease, multiple sclerosis, and other acquired demyelinating syndrome. Mult Scler. 2022;28(10):1553-1561. doi: 10.1177/13524585221081090 [DOI] [PubMed] [Google Scholar]
- 35.Wong YYM, Bruijstens AL, Barro C, et al. Serum neurofilament light chain in pediatric MS and other acquired demyelinating syndromes. Neurology. 2019;93(10):e968-e974. doi: 10.1212/wnl.0000000000008057 [DOI] [PubMed] [Google Scholar]
- 36.Reinert MC, Benkert P, Wuerfel J, et al. Serum neurofilament light chain is a useful biomarker in pediatric multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e749. doi: 10.1212/nxi.0000000000000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhle J, Chitnis T, Banwell B, et al. Plasma neurofilament light chain in children with relapsing MS receiving teriflunomide or placebo: a post hoc analysis of the randomized TERIKIDS trial. Mult Scler. 2023;29(3):385-394. doi: 10.1177/13524585221144742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 40.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 41.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 42.Nitz E, Smitka M, Schallner J, et al. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann Clin Transl Neurol. 2021;8(10):2013-2024. doi: 10.1002/acn3.51449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manouchehrinia A, Piehl F, Hillert J, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol. 2020;7(1):139-143. doi: 10.1002/acn3.50972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosso M, Gonzalez CT, Healy BC, et al. Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis. Ann Clin Transl Neurol. 2020;7(6):945-955. doi: 10.1002/acn3.51060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modvig S, Degn M, Sander B, et al. Cerebrospinal fluid neurofilament light chain levels predict visual outcome after optic neuritis. Mult Scler. 2016;22(5):590-598. doi: 10.1177/1352458515599074 [DOI] [PubMed] [Google Scholar]
- 46.Szilasiova J, Mikula P, Rosenberger J, et al. Plasma neurofilament light chain levels are predictors of disease activity in multiple sclerosis as measured by four-domain NEDA status, including brain volume loss. Mult Scler. 2021;27(13):2023-2030. doi: 10.1177/1352458521998039 [DOI] [PubMed] [Google Scholar]
- 47.Calabresi PA, Arnold DL, Sangurdekar D, et al. Temporal profile of serum neurofilament light in multiple sclerosis: implications for patient monitoring. Mult Scler. 2021;27(10):1497-1505. doi: 10.1177/1352458520972573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziemssen T, Arnold DL, Alvarez E, et al. Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol. 2022;13:852563. doi: 10.3389/fimmu.2022.852563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data related to this article will be made available by request from any qualified investigator for the purpose of replicating results. Persons interested in obtaining data should contact Brenda Marie Huppke (Brenda.Huppke@med.uni-jena.de).