Abstract
Background and Objectives
Early exposure to analgesics and sedatives is a key concern for later learning disorders in children. The hippocampus, a key region for learning and memory, may be selectively affected by exposure to benzodiazepines that are commonly used for sedation, particularly in the neonatal period. In this prospective cohort study, the long-term association of neonatal midazolam exposure, a widely used benzodiazepine in neonatal intensive care, with school age hippocampal growth was examined. Higher-order cognitive function in preterm born children was assessed in relation to hippocampal volumes.
Methods
Very preterm born children underwent MRI to characterize the hippocampus and its subfields and neuropsychological testing. Generalized linear models were used to determine the predictors of 8-year hippocampal volumes. Children were assessed on the Wechsler Abbreviated Scales of Intelligence, Second Edition, and the Wechsler Intelligence Scales for Children, Fifth Edition (WISC-V).
Results
A total of 140 preterm children who were 8 years of age participated, and 25 (18%) were exposed to midazolam as neonates. Reduced hippocampal volumes at age 8 years were associated with neonatal midazolam exposure (B = −400.2, 95% CI −14.37 to −786.03, p = 0.04), adjusting for neonatal clinical care factors. Boys exposed to higher doses of midazolam as neonates had smaller hippocampal volumes (χ2 = 14.4, p = 0.002) compared with nonexposed boys and girls (both, p < 0.03). Analysis of the hippocampal subfields in relation to neonatal midazolam dose revealed that higher doses were associated with smaller volumes of the subiculum (p = 0.008), a hippocampal-cortical relay region implicated in memory processes. Furthermore, smaller school age subiculum volumes predicted significantly lower working memory scores on the WISC-V (B = 0.04, 95% CI 0.01–0.07, p = 0.017).
Discussion
Early midazolam exposure and the association with impaired hippocampal growth seem long-lasting and are most apparent in boys. Alterations in subiculum volumes may underlie hippocampus-dependent memory formation processes in preterm born children exposed to midazolam as neonates.
Introduction
Midazolam, a γ-aminobutyric acid A (GABA-A) receptor agonist, is a benzodiazepine with amnesic properties.1,2 Midazolam is a commonly used intravenous sedative in intensive care units for preterm neonates, infants, and children to manage agitation associated with long-term ventilation or for those undergoing surgery.3 Yet, despite the widespread use of midazolam in pediatric intensive care, evidence suggests that it has neuroapoptotic effects.4 In particular, midazolam is still used in very preterm born neonates despite cautions from evidence suggesting short-term effects on hippocampal development and cognitive ability in the vulnerable developing brain.5 In preclinical models, neonatal exposure to midazolam was associated with impaired hippocampal neurogenesis which lasted into adulthood.6 In turn, the long-term consequences of midazolam exposure in the neonatal period for hippocampal maturation and cognitive ability in preterm born children remain uncertain, despite concerns from families regarding pediatric anesthesia and learning disorders.
The mechanisms of the developmental neurotoxicity of midazolam remain an active area of investigation. The activation of GABA-A receptors by midazolam could result in depolarization and excitatory toxicity in neurons during rapid neuronal proliferation.7 It has been speculated that impairment of mitochondrial integrity and accumulation of reactive oxygen species could contribute toward the neuronal loss and cognitive dysfunction caused by midazolam, isoflurane, and nitrous oxide anesthesia.8 In an animal model, midazolam prevented memory formation through inhibiting long-term potentiation (LTP).9 Potential mechanisms of LTP inhibition include the modulation of GABA-A receptors.
Early exposure to midazolam in the developing brain is associated with later life learning and memory deficits after early exposure.5,10 Midazolam may target the hippocampus, which is vulnerable to different exposures. The hippocampus, located in the medial temporal lobe, comprised specialized subfields, which are functionally and anatomically interconnected.11 Hippocampal subfields include the subiculum, presubiculum, parasubiculum, the cornu ammonis (CA) fields 1–4, and the dentate gyrus (DG) (DG granule cell layers, CA4) and have specialized functions in memory processes.12 The CA2/3 and DG (DG granule cell layers + CA4) are implicated in encoding, learning processes, and memory recall. The CA1 region and subiculum are output structures related to retrieval functions and associated with delayed episodic memory.
Very preterm born children and adults can exhibit reduced hippocampal volumes relative to their term born peers,13-15 particularly in the CA1, CA2/3 regions, DG, and subiculum.16,17 Furthermore, worse working memory ability in preterm born children and adults was associated with CA fields (CA 1/2/3), DG, and subiculum volumes.
The long-term effects of benzodiazepine exposure on the developing brain and functional outcomes remain poorly understood. In the current work, we examined the macrostructural development of the hippocampal subfields in a longitudinal study of preterm born children followed since birth to age 8 years. Our central hypothesis was that the macrostructural growth of the hippocampus at school age would continue to demonstrate an association with neonatal midazolam exposure. We first assessed the association among the neonatal volumes and the 8-year volumes to determine whether the volumes followed a typical developmental trajectory. We then assessed the macrostructure of the hippocampus in the ninth year of life in relation to neonatal midazolam exposure. It was hypothesized that neonatal midazolam exposure would be associated with smaller volumes of the hippocampus at school age. Children underwent MRI scanning to obtain high-resolution anatomical images and the whole hippocampus volumes, and its subfields were automatically extracted. Given the known associations between the CA fields (CA1, CA2/3), subiculum, and DG and working memory abilities in the preterm population,16 we restricted our search to these areas. Using these data, we assessed 8-year hippocampal subfield (CA fields, subiculum, and DG) volumes in relation to neonatal midazolam dose.18 We hypothesized that higher neonatal midazolam doses would be associated with smaller hippocampal subfield volumes. Finally, we assessed the development of the hippocampal subfields in relation to full-scale IQ and working memory ability in the school age cohort. Children underwent neuropsychological testing at the same time as the MRI scanning session. We subsequently compared the hippocampal subfields, focusing on our predefined areas of interest, in relation to full-scale IQ and working memory ability. It was hypothesized that the hippocampal subfields would be associated with IQ and working memory ability in children born preterm.
Methods
Participants
Preterm born children (n = 234) were originally enrolled in a larger longitudinal study that examined the long-term effects of neonatal pain-related stress on the neurodevelopment of children born very preterm (24–32 weeks' gestation), who were admitted to the Level III neonatal intensive care unit (NICU) at British Columbia's Women's Hospital from 2006 to 2013.19 Data from a subset of the neonatal cohort were reported previously.20 A total of 142 preterm born children returned for MRI scans and neuropsychological follow-up testing at the University of British Columbia (2015–2022). None of the children had a major sensory or motor impairment diagnosed at age 8 years. Psychological testing was conducted by highly experienced psychology staff in the Neonatal Follow-up Program at BC Women's Hospital.
Standard Protocol Approvals, Registrations, and Patient Consents
The Clinical Research Ethics Board of the University of British Columbia and the British Columbia Children's and Women's Research Ethics Board approved the study. Parents provided consent and children provided assent to participate in the studies.
Clinical Data Collection
Review of neonatal data from birth to term equivalent was performed by neonatal clinical research nurses. Data collected included gestational age, days of mechanical ventilation, number of skin-breaking procedures, surgeries, and cumulative doses of morphine, fentanyl, midazolam, and steroids (hydrocortisone, dexamethasone). The cumulative doses were calculated (intravenous dose plus converted oral dose) as the average daily dose in milligrams adjusted for daily body weight in kilograms, multiplied by the number of days the drug was administered.20
MRI
Neonatal scanning occurred without pharmacologic sedation on a Siemens (Erlangen, Germany) on a 1.5 T Avanto MRI using an MR-conditional incubator (Lammers Medical Technology, Luebeck, Germany) and a single-channel neonatal head coil (Advanced Imaging Research, Cleveland, OH). MRI scans were performed as soon the neonate was clinically stable for transport within the first few weeks of life (median age 32 weeks, interquartile range [IQR] 30–34 weeks) and again at term-equivalent age (median age 40.14 weeks, IQR 38.7–42). Anatomical imaging included T1-weighted images that were acquired using coronal or sagittal sequences: repetition time [TR], 36; echo time [TE], 9.2; field of view [FOV], 192 × 88 mm; voxel size: 1.04 × 1.00 × 1.04 mm; and no gap. For the neuroradiologic assessment of brain injury, T2-weighted images were acquired using an axial fast spin echo (TR = 4610; TE = 107; FOV = 20 × 402 mm; voxel size = 0.4 × 0.4 × 4.48 mm; gap = 0.2 mm). At age 8 years, MRI was performed using a 32-channel head coil on a GE Medical Systems 3 Tesla scanner (Discovery MR750). To study the volumetric organization of the developing brain at age 8 years, children underwent a 3-dimensional high-resolution T1-weighted fast spoiled gradient-echo scan using the following parameters: TR/TE/flip angle = 8.2 milliseconds/3.2 milliseconds/12°, FOV = 256 × 256, 190 slices, slice thickness = 0.9 mm, and isotropic voxels.
Hippocampal Segmentation
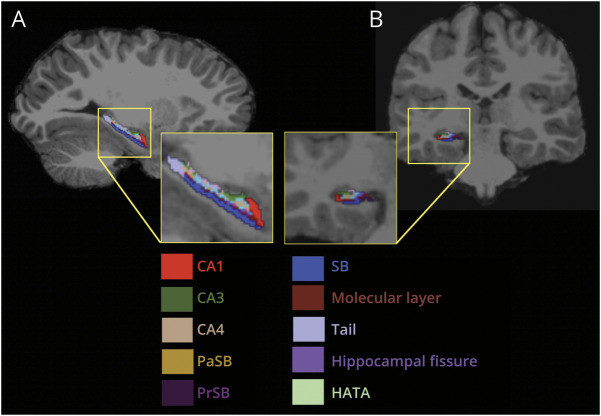
FreeSurfer version 6.0 was used to segment and isolate the hippocampal subfields. The entire hippocampus was segmented, bilaterally, into the hippocampal tail, subiculum, CA1, hippocampal fissure, presubiculum, parasubiculum, molecular layer, granule cell (GC) layers of the DG, CA3, CA4, fimbria, and the hippocampus-amygdala transition area (Figure 1). The total cerebral volumes were also extracted using the FreeSurfer pipeline. The hippocampal segmentations were visually inspected before inclusion using Freeview software, which is available within the FreeSurfer suite of tools.
Figure 1. Hippocampal Subfield Segmentation of a T1-Weighted Image in a Representative Participant.
(A) Sagittal view of the hippocampus. (B) Coronal view of the hippocampus. CA = cornu ammonis; HATA = hippocampus-amygdala transition area; SB = subiculum; PaSB = parasubiculum; PrSB = presubiculum.
Neuropsychological Testing: 8 Years
Children were assessed on neuropsychological tests by a trained clinical psychologist at age 8 years, who was blinded to the children's midazolam exposure in the neonatal period. Cognitive ability was determined using the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V).21 Children were assessed only on the working memory subtests (i.e., digit span, picture span). Full-scale IQ at 8 years was assessed using the 2-subtest version (e.g., vocabulary, matrix reasoning) of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II).22 The WISC-V and the WASI-II are standardized tests of intelligence. The mean score is 100, and the standard deviation is 15. Maternal education was based on self-report and was categorized as primary school or high school, college, or university undergraduate degree or postgraduate studies.
Statistical Analyses
Analyses were conducted using Statistical Package for the Social Sciences (version 28; SPSS, Inc., Chicago, IL). As part of an exploratory aim, we examined the associations among the neonatal volumes acquired within the first weeks after birth and term-equivalent age, with 8-year hippocampal volumes using a generalized linear model. To address our first aim as to whether neonatal midazolam exposure would predict 8-year hippocampal volumes, in a basic model, neonatal midazolam exposure (exposed vs non exposed) in relation to 8-year whole hippocampal volumes was assessed in a generalized linear model, adjusting for age, sex, neonatal clinical variables including gestational age, days of mechanical ventilation, surgery, positive infection, exposure to other analgesic and sedative medications including morphine and fentanyl, neonatal skin-breaking procedures (i.e., heel lances, chest intubations), steroids (hydrocortisone), and total cerebral volumes. As we had 1 a priori hypothesis that midazolam would be associated with volumetric disturbances in the hippocampus at age 8 years, we set the alpha level at 0.05. Significant main effects were examined with subsequent interaction analyses, and pairwise analyses were performed on significant main effects. The pairwise comparisons were Bonferroni-corrected for multiple comparisons.
In our second aim, we subsequently assessed hippocampal subfield volumes in relation to midazolam dose adjusting for the same covariates. We examined the summed totals of the left and right subiculum, CA fields (CA1, CA2/3), and DG (GC, dentate gyrus)18 as the dependent variables in 3 separate generalized linear models, with midazolam exposure as a factor and clinical and demographic variables as covariates. As we had 3 separate hypotheses for each of the hippocampal subfield, the alpha level was set at p = 0.017 (p = 0.05/3).
To address our third aim, we assessed the association of hippocampal subfield volumes in the ninth year of life with the IQ scores on the WASI-II and the working memory composite scores on the WISC-V, adjusting for maternal education, biological sex, age at scan, and total cerebral volumes. Scores on the WISC-V are standardized with a mean of 100 and a standard deviation of 15. As we examined hippocampal subfield volumes in relation to full-scale IQ and the working memory subtests of the WISC-V in 2 separate models, we set the alpha level at p = 0.025 (p = 0.05/2).
Data Availability
Families were not consented for data sharing, and for ethical reasons, the data cannot be shared.
Results
Demographic Information
Of the 142 children examined at age 8 years, a total of 140 (99%) successfully completed the MRI scans and had quality MRI data (79 boys [56%]) and 25 (18%) were exposed to midazolam as neonates. Participant demographics are listed in Table 1. No significant differences in neonatal clinical variables, including gestational age at birth, total neonatal skin-breaking procedures, total doses of morphine, midazolam, fentanyl, and days spent on mechanical ventilation, were evident between the children who returned at 8 years compared with those who were enrolled initially (all, p > 0.1).
Table 1.
Participant Characteristics
| Characteristic | Neonatal midazolam exposure (n = 25) | No exposure to neonatal midazolam (n = 115) | p Value |
| Birth GA (wk), median (IQR) | 25.4 (24.9–26.1) | 28.3 (27.4–28.8) | <0.001a |
| Male, n (%) | 15 (60) | 57 (50) | 0.9 |
| Age at scan (mo) | 98.3 (96.4–103.3) | 97.6 (96.4–35.100.6) | 0.1 |
| Days of mechanical ventilation, median (IQR) | 57 (41–68) | 2 (0–9) | <0.001a |
| Culture positive infection, n (%) | 23 (92) | 36 (37.1) | <0.001a |
| Midazolam,b no. of exposed | 14, 25 | — | — |
| Median dose (IQR) in exposed | (0–4.09) | — | — |
| Hydrocortisone,b no. of exposed | 30, 15 | 6.73, 10 | <0.001a |
| Median dose (IQR) in exposed | (10.95–62.4) | (1.6–11.8) | |
| Fentanyl,b no. of exposed | 0.02, 16 | 0.01, 17 | 0.9 |
| Median dose (IQR) in exposed | (0.007–0.05) | (0.005–0.009) | |
| Morphine,b no.of exposed | 0.17, 25 | 0.96, 60 | <0.001a |
| Median dose (IQR) in exposed | (4.9–56.2) | (0.2–13.9) | |
| Surgeries, n (%) | 19 (76) | 21 (18.3) | <0.001a |
| Total invasive procedures, median (IQR) | 236 (177.5–286) | 88 (53.3–115.75) | <0.001a |
| Maternal education categoriesc | 5 (5–6) | 5 (5–6) | 0.6 |
Abbreviations: GA = gestational age; IQR = interquartile range; PMA = postmenstrual age.
Probability values provide results using t test for continuous measures and χ2 tests for categorical measures comparing neonates who had no neonatal midazolam exposure vs those who were exposed to midazolam.
Statistically significant, p < 0.05.
Cumulative dose (in milligrams) adjusted for daily body weight.
Hollingshead category 5 is college and 6 university level.
Neonatal Brain Injury
MRI scans acquired in the neonatal period were reviewed by a neuroradiologist. A total of 42 (30%) neonates had mild/moderate white matter injury, 59 (42%) had mild and 5 (4%) had severe intraventricular hemorrhage, and 18 (13%) had evidence of cerebellar hemorrhage.
Relationship Between School Age Hippocampal Volumes and Neonatal Hippocampal Growth
As part of an exploratory aim, we examined whether neonatal hippocampal volumes predicted the volumes of the hippocampus assessed in preterm born children. The neonatal hippocampal volumes were extracted from T1-weighted images acquired first few weeks of life (median age 32, IQR 30–34) and at term-equivalent age (median age 40.7, IQR 39.1–44) using previously described methods.5 Using a generalized linear model, neonatal volumes at term-equivalent age (B = 0.63, p < 0.001) positively predicted 8-year hippocampal volumes, adjusting for biological sex, age at 8-year assessment, and 8-year total cerebral volumes. Volumes acquired in the initial weeks of life, before term-equivalent age, were not associated with 8-year hippocampal volumes (B = 0.3, p = 0.2).
Relationship Between Hippocampal Volumes and Neonatal Midazolam Exposure
The association between 8-year hippocampal volumes and neonatal midazolam exposure was examined in a generalized linear model. Neonatal midazolam exposure was associated with smaller whole volumes of the hippocampus at age 8 years (B = −400.2, p = 0.04; Table 2, Figure 2A). Biological sex was also significant in the model (B = 313.6, p = 0.007). In a subsequent interaction analysis (sex × midazolam exposure [χ2 = 14.4, p = 0.002]), boys exposed to midazolam in the neonatal period were found to have smaller hippocampal volumes when compared with nonexposed boys (p = 0.03; Figure 2B) and nonexposed girls (p = 0.007), whereas the volumes were not significantly different from exposed girls (p = 0.25), Bonferroni-corrected for multiple comparisons. Positive culture infection was also significant in the main model, and a subsequent interaction analysis was performed. Neonatal midazolam exposure and positive culture infection demonstrated a significant interaction (χ2 = 9.59, p = 0.02). Children who had been exposed to neonatal midazolam and who had a positive culture infection in the neonatal period had smaller hippocampal volumes in comparison with children who did not have a positive culture infection or were exposed to neonatal midazolam (p = 0.016). The residuals of the main model were assessed for normality using the Kolmogorov-Smirnov test. The residual from 1 participant was considered an outlier. Removal of this participant's data from the original model resulted in comparable effects sizes, indicating that the results were not unduly influenced by this participant.
Table 2.
Results of Generalized Linear Model of 8-Year Whole Hippocampal Volumes in All Participants Examining the Association With Neonatal Midazolam Exposure

| Characteristics | B | 95% CI | p Value |
| 8 y ages (mo) | −1.74 | −8.95 to 5.47 | 0.64 |
| Gestational age at birth (wk) | 20.61 | −54.53 to 95.74 | 0.59 |
| Sex (male) | 313.6 | 83.88 to 543.33 | 0.007a |
| Culture positive infection | −320.5 | −603.2 to −37.8 | 0.03a |
| Surgery ≥1 | −78.6 | −428.72 to 271.51 | 0.66 |
| Midazolam exposure | −400.2 | −14.37 to −786.03 | 0.04a |
| Fentanyl doseb | −15.0 | −92.7 to 62.69 | 0.71 |
| Morphine doseb | −2.57 | −8.16 to 3.02 | 0.37 |
| Hydrocortisoneb | 1.90 | −7.91 to 11.7 | 0.70 |
| Days of mechanical ventilation | −1.23 | −9.75 to 7.29 | 0.78 |
| Total skin-breaking proceduresc | 2.72 | −0.09 to 5.53 | 0.06 |
| TCV | 0.0001 | 0.001 to 0.002 | <0.001a |
| Interaction analysis | χ2 | 95% CI | p Value |
| Sex × midazolam exposured | 14.4 | — | 0.002 |
| Pairwise comparisons | Mean difference | 95% CI | p Value |
| Male × midazolam − | 654 | 30.2 to 1,277.5 | 0.03 |
| Female × midazolam − | 413 | 77.7 to 747.5 | 0.007 |
| Female × midazolam + | 521 | −151.2 to 1,193.4 | 0.25 |
| Male × midazolam +e | — | — | — |
Abbreviations: GA = gestational age; TCV = total cerebral volume.
Midazolam exposure (+) compared with no exposure (−) to midazolam in the neonatal period. Neonatal midazolam exposure was assessed in boys and girls in an interaction analysis, followed by pairwise comparisons (Bonferroni-corrected for multiple comparisons).
Statistically significant, p < 0.05.
Total dose in milligrams from birth until hospital discharge.
Total invasive procedures from birth until hospital discharge.
Adjusting for the same clinical variables as the main model.
Baseline comparison.
Figure 2. Neonatal Midazolam Exposure and the Association With 8-Year Hippocampal Volumes.
(A) 8-year hippocampal volumes in relation to exposure (+) to neonatal midazolam compared with no exposure (−). *p < 0.05. The results are Bonferroni-corrected for multiple comparisons. Values reflect the estimated marginal means of whole hippocampal volumes at age 8 years in children who were exposed (+) vs nonexposed (−) to neonatal midazolam, adjusting for age, sex, neonatal clinical variables including gestational age, days of mechanical ventilation, surgery, positive infection, exposure to other analgesic and sedative medications including morphine and fentanyl, neonatal skin-breaking procedures, steroids, and total cerebral volumes. Error bars are the standard errors. (B) Whole hippocampal volumes in preterm born boys who were exposed (+) to neonatal midazolam compared with unexposed (−) boys and girls. *p < 0.05. The results are Bonferroni-corrected for multiple comparisons. Values reflect the estimated marginal means of whole hippocampal volumes at age 8 years, adjusting for clinical and demographic variables. *p < 0.05, **p < 0.01. Error bars are the standard errors.
Relationship Between Hippocampal Subfield Volumes and Neonatal Midazolam Dose
In a subsequent generalized linear model to address our second aim, the subiculum volumes were significantly negatively predicted by neonatal midazolam dosage (B = −2.9, p = 0.008; Table 3, Figure 3) when adjusting for sex and age as well as clinical care factors including skin-breaking procedures, days of mechanical ventilation, surgery, analgesics, sedatives (morphine, fentanyl), steroids, and total cerebral volumes. CA fields and DG volumes were not associated with midazolam dose (both, p > 0.5).
Table 3.
Results of Generalized Linear Model of 8-Year Subiculum Volumes in All Participants Examining the Association With Total Neonatal Midazolam Dose

| Characteristics | B | 95% CI | p Value |
| 8 y ages (mo) | −0.02 | −0.87 to 0.84 | 0.97 |
| Gestational age at birth (weeks) | 1.72 | −7.505 to 10.95 | 0.72 |
| Sex (male) | 29.18 | 0.941 to 57.41 | 0.04a |
| Culture-positive infection | −38.52 | −73.01 to −4.03 | 0.03a |
| Surgery ≥1 | −28.03 | 70.134 to −14.06 | 0.19 |
| Fentanyl doseb | −1.81 | −11.352 to 7.73 | 0.71 |
| Morphine doseb | −0.05 | −0.73 to 0.63 | 0.89 |
| Midazolam doseb | −2.93 | −5.105 to −0.75 | 0.008a |
| Hydrocortisone doseb | −0.17 | −1.353 to 1.02 | 0.79 |
| Days of mechanical ventilation | −0.23 | −1.228 to 0.76 | 0.65 |
| Total skin-breaking proceduresc | 0.38 | 0.029 to 0.72 | 0.03a |
| TCV | 0.0002 | 0.0001 to 0.0003 | <0.001a |
Abbreviations: GA = gestational age; TCV = total cerebral volume.
Statistically significant, p < 0.05.
Total dose in milligrams from birth until hospital discharge.
Total invasive procedures from birth until hospital discharge.
Figure 3. Eight-Year Subiculum Volumes (mm3) in Relation to Neonatal Midazolam Total Dose (Lg10).
Relationship Between School Age Hippocampal Subfield Volumes and Cognitive Ability
Based on the findings that the subiculum was selectively associated with midazolam exposure, we examined its volume in relation to full-scale IQ using the WASI-II (mean 102, IQR 92–114, range 71–140) and with working memory ability assessed on the WISC-V (mean 97, IQR 85–110, range 15–135, eFigure 1, links.lww.com/WNL/D116) using generalized linear models.
The results indicated that the working memory composite scores, comprised of the Digit Span and Picture Span subscales, were significantly predicted by 8-year subiculum volumes (B = 0.04, p = 0.017; Table 4), adjusting for sex (B = −3.7, p = 0.15), age at assessment (B = −0.01, p = 0.7), and maternal education (B = 2.16, p = 0.07). In 2 subsequent exploratory analyses, the subscales of the Working Memory Composite were examined in generalized linear models. When examining the subtests, Digit Span (auditory short-term memory) was significantly associated with subiculum volumes (B = 0.009, p = 0.002), whereas Picture Span (visual short-term memory) was not (B = 0.004, p = 0.2). Full-scale IQ scores were not associated with subiculum volumes (B = 0.03, p = 0.04), when adjusting for multiple comparisons.
Table 4.
Results of Generalized Linear Model of 8-Year WISC-V Working Memory Subtest Scores

| Characteristic | B | 95% CI | p Value |
| 8 y ages (mo) | −0.03 | −0.19, 0.13 | 0.71 |
| Gestational age at birth (wk) | 0.75 | −0.34, 1.84 | 0.18 |
| Sex (male) | −3.74 | −8.76, 1.29 | 0.15 |
| Subiculum volumes (mm3) | 0.04 | 0.01, 0.07 | 0.017a |
| Maternal education level | 2.16 | −0.19, 4.50 | 0.07 |
Abbreviation: WISC-V = Wechsler Intelligence Scale for Children, Fifth Edition.
Statistically significant, p < 0.05.
Discussion
The hippocampus is a brain structure recognized to be selectively vulnerable to the harmful effects of stress.23 The hippocampus is enriched with glucocorticoid receptors and plays a key role in the hypothalamic-pituitary-adrenal axis, the body's stress response system.24 Evidence from both animal and human studies has provided corroborative evidence that stress is associated with the release of corticosteroids that can selectively target the hippocampus.25 Decreased macrostructural growth of the hippocampus has been associated with cognitive abilities in children.26,27 In this work, through the continued follow-up of a neonatal cohort from birth until 8 years of age with MRI and neuropsychological follow-up testing, we demonstrate lasting associations of neonatal midazolam exposure with hippocampal volumes. In the neonatal cohort, hippocampal volumes were associated with midazolam dose. We further report an association of hippocampal volumes at term age with 8-year hippocampal volumes. Furthermore, boys exposed to midazolam in the neonatal period showed enhanced hippocampal vulnerability, a finding not recognized at the earlier age of follow-up.5 We report an association between subiculum subfields and neonatal midazolam dose, whereby preterm born children exposed to higher doses of neonatal midazolam had smaller volumes of the subiculum at age 8 years. Furthermore, smaller subiculum volumes predicted working memory abilities at school age. Findings suggest that neonatal midazolam may have long-term effects, beyond the NICU stay, and may be a risk factor for altered development of the hippocampus and subsequent memory impairments in children born preterm.
Anterograde amnesia is a well-documented and robust phenomenon described in the literature in both young children and adults exposed to midazolam.28,29 The use of midazolam is favored to minimize stress and anxiety before a surgery as a sedative premedication30 as it may mitigate adverse behavioral changes associated with analgesic and sedative practices seen in children. After short-term midazolam exposure, recall and recognition memory was impaired in comparison with postoperative children who had not received midazolam.31,32
Although the mechanisms of anterograde amnesia after midazolam exposure have yet to be described, animal studies have indicated that midazolam results in structural remodeling of the hippocampus. Midazolam injection into the hippocampus prevents memory formation through inhibiting long-term potentiation.33 Whether delayed maturation of the hippocampus in the first 8 years of life is a result of impaired LTP in the neonatal period is beyond the scope of the current work. Future work in this area is warranted to better understand the mechanistic action of midazolam on early-life brain function and subsequent maturation of the hippocampus to school age. Our results regarding the association between neonatal midazolam exposure and hippocampal volumes were maintained when adjusting for several clinical care factors in the neonatal period including exposure to steroids, which have been associated with alterations in brain development in infants34 as well as children and adolescents born preterm,35 whereas others have reported no association.36
Boys exposed to higher doses of midazolam had smaller hippocampal volumes in the neonatal period and at school age in comparison with girls, adjusting for total cerebral volumes. Although the findings were obtained in a small sample of boys, evidence of a dose-related response in hippocampal volumes is contributing to a growing body of evidence that pain management practices in the neonatal period may have differential effects for boys and girls.37,38 Previous research with adults has noted differential responses to midazolam administration between boys and girls.39 In particular, in female preterm born neonates, exposure to pain predicts slower growth of the brain and subcortical structures relative to this exposure in male preterm born neonates.37 Sex-based differences in cortical responses to noxious stimuli have been reported in neonates using EEG40 with boys who demonstrating localized activation and girls demonstrating widespread activation. Behavioral responses to pain including facial expressions and crying in response to pain were greater in girls compared with boys.41 An additional consideration is that GABA receptor agonists, including midazolam, propofol, and dexmedetomidine, are commonly used sedative agents in critical care. These receptor agonists are sympatholytic agents and can have a major influence on the stress response system, which may affect girls and boys differently.42 The interaction of neonatal infection and midazolam exposure indicates that the association of midazolam exposure with growth of the hippocampus is complex and modified by other clinical exposures; whether this interaction is mediated by inflammatory mechanisms warrants further attention. Findings highlight the importance of the long-term study of sex-based differences when examining pain management practices and other clinical exposures that may contribute to brain maturation in childhood, particularly in boys.
Previous research examining long-term outcomes of very preterm born neonates at school age and adulthood has indicated significant cognitive deficits in comparison with term born participants.43 Preterm children also have lower measures of immediate, working, and long-term memory compared with term born children.44 Our findings regarding the association of hippocampal development and cognitive outcomes are in line with previous research with older children and adolescents demonstrating alterations in both hippocampal development and their working memory abilities (for review, see Reference 15). Several studies have examined child and adolescent hippocampal subfield volumes and working memory abilities16,17; however, one study with 9-year-old preterm born children noted decreased volumes of the hippocampus but no impairments with episodic memory.45 The authors noted that the preterm children in that study did not have complicated clinical courses in the neonatal period. In turn, a contributing factor to the optimal findings for the memory outcomes may be early neonatal clinical care. Our results demonstrating a dose-dependent response of midazolam on subiculum subfield volumes may also point to selective vulnerability of hippocampal subfields to benzodiazepines in the neonatal period.
The subiculum is an elongated structure and is the most inferior subfield of the hippocampus, which is located between the entorhinal cortex and the CA1 subfield. This region is involved in mnemonic processes and is also a regulator of the hypothalamic-pituitary-adrenal axis involved in stress responses. The subiculum receives inputs from the entorhinal, perirhinal, and prefrontal cortices and sends reciprocal projections to these structures.46 In addition, this region contains benzodiazepine/GABAA receptors,47 indicating this region may be vulnerable to sedative exposure. In preterm born children, lower volumes of this structure have been reported.16 Given the connectivity patterns of the subiculum to key regions involved in memory processes, the subiculum has been implicated in verbal and visual episodic memory.48 Higher doses of midazolam in the neonatal period may be associated with alterations in the density and organization of the benzodiazepine/GABAA receptors in the subiculum, which could later be reflected in macrostructural changes and subsequent impacts on mnemonic processes.
An important consideration is that many survivors of the NICU who go on to escape major deficits may demonstrate difficulties with more subtle cognitive functions that only appear at school age when tested,49 indicating that more sensitive tests probing executive functions such as working memory are needed to fully assess the array of cognitive deficits seen in the preterm population.
Midazolam is a short acting sedative that is variably used in intensive care units with infants and children. The results of this longitudinal study of preterm born children demonstrated an association between school age hippocampal volumes and neonatal midazolam dosage. While examination of the direct effects of neonatal midazolam on brain development and outcome was not possible for our study design, future research using epidemiologic approaches would be warranted. In our models, we adjusted for factors related to clinical care in the neonatal period including exposure to steroids and surgeries. While known clinical factors were adjusted for in our analyses, there may have been other factors that were not examined in the current work such as bronchopulmonary dysplasia or necrotizing enterocolitis, which may have influenced brain morphology. Based on analyses, our results indicate that midazolam may have long-lasting effects on subiculum volumes. Furthermore, alterations in the development of the subiculum were associated with worse auditory working memory ability at school age. Our findings of altered hippocampal volumes in boys exposed to midazolam highlights the importance of examining sex-based differences in the context of pain management practices and the long-term follow-up of this vulnerable group of children.
Acknowledgment
The sincerely thank the families that participated in this research. Janet Rigney, Sandra Belanger (RN), and Mark Chalmers (RRT) were instrumental to the data collection, Ivan Cepeda for the database development, and Dr. Kenneth Poskitt for neuroradiology review of the neonatal MRI scans.
Glossary
- CA
cornu ammonis
- DG
dentate gyrus
- FOV
field of view
- GABA-A
γ-aminobutyric acid A
- IQR
interquartile range
- LTP
long-term potentiation
- NICU
neonatal intensive care unit
- TE
echo time
- TR
repetition time
- WASI-II
Wechsler Abbreviated Scale of Intelligence, Second Edition
- WISC-V
Wechsler Intelligence Scales for Children, Fifth Edition
Appendix. Authors

| Name | Location | Contribution |
| Emma G. Duerden, PhD | Western University, London, Ontario, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Ting Guo, PhD | Hospital for Sick Children, Toronto, Ontario, Canada | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of the data |
| Cecil Chau, MSc | University of British Columbia, Vancouver, Canada | Major role in the acquisition of data |
| Vann Chau, MD | The Hospital for Sick Children and University of Toronto, Ontario, Canada | Major role in the acquisition of data; study concept or design |
| Anne Synnes, MDCM | University of British Columbia, Vancouver, Canada | Major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Ruth E. Grunau, PhD | University of British Columbia, Vancouver, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Steven P. Miller, MDCM, MAS | University of British Columbia, Vancouver, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
This research has been funded by the Canadian Institutes of Health Research (CIHR) MOP 79262 to S.P. Miller and MOP 86489 to R.E. Grunau and the Kid's Brain Health Network to S.P. Miller.
Disclosure
S.P. Miller was supported by the Bloorview Children's Hospital Chair in Paediatric Neuroscience and now by the Hudson Family Hospital Chair in Pediatric Medicine and the James & Annabel McCreary Chair in Pediatrics. All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Polster MR, Gray PA, O'Sullivan G, McCarthy RA, Park GR. Comparison of the sedative and amnesic effects of midazolam and propofol. Br J Anaesth. 1993;70(6):612-616. doi: 10.1093/bja/70.6.612 [DOI] [PubMed] [Google Scholar]
- 2.Veselis RA, Reinsel RA, Feshchenko VA, Wroński M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87(4):749-764. doi: 10.1089/brain.2012.0107 [DOI] [PubMed] [Google Scholar]
- 3.Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2017;1:CD002052. doi: 10.1002/14651858.CD002052.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberger DS, Falangola MF, Ledreux A, et al. Memory and hippocampal architecture following short-term midazolam in western diet-treated rats. Neurosci Lett. 2016;621:68-74. doi: 10.1016/j.neulet.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duerden EG, Guo T, Dodbiba L, et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol. 2016;79(4):548-559. doi: 10.1002/ana.24601 [DOI] [PubMed] [Google Scholar]
- 6.Doi H, Matsuda T, Sakai A, et al. Early-life midazolam exposure persistently changes chromatin accessibility to impair adult hippocampal neurogenesis and cognition. Proc Natl Acad Sci USA. 2021;118(38):e2107596118. doi: 10.1073/pnas.2107596118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002;36(6):989-991. doi: 10.1016/s0896-6273(02)01136-4 [DOI] [PubMed] [Google Scholar]
- 8.Boscolo A, Milanovic D, Starr JA, et al. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology. 2013;118(5):1086-1097. doi: 10.1097/ALN.0b013e318289bc9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuda K, O'Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010;30(50):16788-16795. doi: 10.1523/JNEUROSCI.4101-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Niu M, Bai S. Effects of long-term infusion of sedatives on the cognitive function and expression level of RAGE in hippocampus of rats. J Anesth. 2016;30(4):691-695. doi: 10.1007/s00540-016-2192-3 [DOI] [PubMed] [Google Scholar]
- 11.Vos de Wael R, Larivière S, Caldairou B, et al. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc Natl Acad Sci USA. 2018;115(40):10154-10159. doi: 10.1073/pnas.1803667115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15(5):333-351. doi: 10.1515/revneuro.2004.15.5.333 [DOI] [PubMed] [Google Scholar]
- 13.Aanes S, Bjuland KJ, Skranes J, Løhaugen GC. Memory function and hippocampal volumes in preterm born very-low-birth-weight (VLBW) young adults. Neuroimage. 2015;105:76-83. doi: 10.1016/j.neuroimage.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 14.Hedderich DM, Avram M, Menegaux A, et al. Hippocampal subfield volumes are nonspecifically reduced in premature-born adults. Hum Brain Mapp. 2020;41(18):5215-5227. doi: 10.1002/hbm.25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosarti C, Froudist-Walsh S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev Med Child Neurol. 2016;58(suppl 4):35-45. doi: 10.1111/dmcn.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aanes S, Bjuland KJ, Sripada K, et al. Reduced hippocampal subfield volumes and memory function in school-aged children born preterm with very low birthweight (VLBW). Neuroimage Clin. 2019;23:101857. doi: 10.1016/j.nicl.2019.101857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández de Gamarra-Oca L, Zubiaurre-Elorza L, Junqué C, et al. Reduced hippocampal subfield volumes and memory performance in preterm children with and without germinal matrix-intraventricular hemorrhage. Sci Rep. 2021;11(1):2420. doi: 10.1038/s41598-021-81802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller SG, Yushkevich PA, Das S, et al. Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage Clin. 2018;17:1006-1018. doi: 10.1016/j.nicl.2017.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean MA, Scoten OC, Chau CMY, Synnes A, Miller SP, Grunau RE. Association of neonatal pain-related stress and parent interaction with internalizing behaviors across 1.5, 3.0, 4.5, and 8.0 years in children born very preterm. JAMA Netw Open. 2022;5(10):e2238088. doi: 10.1001/jamanetworkopen.2022.38088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385-396. doi: 10.1002/ana.22267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Intelligence Scale for Children. 5th ed. NCS Pearson; 2014. [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II). NCS Pearson; 2011. [Google Scholar]
- 23.McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353-1363. doi: 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118-134. doi: 10.1210/edrv-12-2-118 [DOI] [PubMed] [Google Scholar]
- 25.Joëls M. Corticosteroids and the brain. J Endocrinol. 2018;238(3):R121-R130. doi: 10.1530/JOE-18-0226 [DOI] [PubMed] [Google Scholar]
- 26.Tamnes CK, Walhovd KB, Engvig A, et al. Regional hippocampal volumes and development predict learning and memory. Dev Neurosci. 2014;36(3-4):161-174. doi: 10.1159/000362445 [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Ekstrom AD, Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage. 2014;94:162-171. doi: 10.1016/j.neuroimage.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 28.Twersky RS, Hartung J, Berger BJ, McClain J, Beaton C. Midazolam enhances anterograde but not retrograde amnesia in pediatric patients. Anesthesiology. 1993;78(1):51-55. doi: 10.1097/00000542-199301000-00009 [DOI] [PubMed] [Google Scholar]
- 29.De Roode A, Jelicic M, Bonke B, Bovill JG. The effect of midazolam premedication on implicit memory activation during alfentanil-nitrous oxide anaesthesia. Anaesthesia. 1995;50(3):191-194. doi: 10.1111/j.1365-2044.1995.tb04553.x [DOI] [PubMed] [Google Scholar]
- 30.Kain ZN, Sevarino F, Pincus S, et al. Attenuation of the preoperative stress response with midazolam: effects on postoperative outcomes. Anesthesiology. 2000;93(1):141-147. doi: 10.1097/00000542-200007000-00024 [DOI] [PubMed] [Google Scholar]
- 31.Stewart SH, Buffett-Jerrott SE, Finley GA, Wright KD, Valois Gomez T. Effects of midazolam on explicit vs implicit memory in a pediatric surgery setting. Psychopharmacology (Berl). 2006;188(4):489-497. doi: 10.1007/s00213-006-0402-7 [DOI] [PubMed] [Google Scholar]
- 32.Viana KA, Daher A, Maia LC, et al. What is the level of evidence for the amnestic effects of sedatives in pediatric patients? A systematic review and meta-analyses. PLoS One. 2017;12(7):e0180248. doi: 10.1371/journal.pone.0180248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans MS, Viola-McCabe KE. Midazolam inhibits long-term potentiation through modulation of GABAA receptors. Neuropharmacology. 1996;35(3):347-357. doi: 10.1016/0028-3908(95)00182-4 [DOI] [PubMed] [Google Scholar]
- 34.Thompson DK, Wood SJ, Doyle LW, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642-651. doi: 10.1002/ana.21367 [DOI] [PubMed] [Google Scholar]
- 35.Cheong JL, Burnett AC, Lee KJ, et al. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J Pediatr. 2014;164(4):737-743.e1. doi: 10.1016/j.jpeds.2013.10.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strahle JM, Triplett RL, Alexopoulos D, et al. Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. Neuroimage Clin. 2019;22:101787. doi: 10.1016/j.nicl.2019.101787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider J, Duerden EG, Guo T, et al. Procedural pain and oral glucose in preterm neonates: brain development and sex-specific effects. Pain. 2018;159(3):515-525. doi: 10.1097/j.pain.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 38.Rothstein S, Simkins T, Nuñez JL. Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience. 2008;152(4):959-969. doi: 10.1016/j.neuroscience.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster A, Gardaz JP, Suter PM, Gemperle M. I.V. midazolam as an induction agent for anaesthesia: a study in volunteers. Br J Anaesth. 1980;52(9):907-911. doi: 10.1093/bja/52.9.907 [DOI] [PubMed] [Google Scholar]
- 40.Verriotis M, Jones L, Whitehead K, et al. The distribution of pain activity across the human neonatal brain is sex dependent. Neuroimage. 2018;178:69-77. doi: 10.1016/j.neuroimage.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller BF. Infant gender differences regarding acute established pain. Clin Nurs Res. 2002;11(2):190-203. doi: 10.1177/105477380201100207 [DOI] [PubMed] [Google Scholar]
- 42.Bangasser DA, Wiersielis KR. Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones (Athens). 2018;17(1):5-13. doi: 10.1007/s42000-018-0002-z [DOI] [PubMed] [Google Scholar]
- 43.Løhaugen GC, Gramstad A, Evensen KA, et al. Cognitive profile in young adults born preterm at very low birthweight. Dev Med Child Neurol. 2010;52(12):1133-1138. doi: 10.1111/j.1469-8749.2010.03743.x [DOI] [PubMed] [Google Scholar]
- 44.Omizzolo C, Scratch SE, Stargatt R, et al. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory. 2014;22(6):605-615. doi: 10.1080/09658211.2013.809765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunnemann N, Kipp KH, Gortner L, et al. Alterations in the relationship between hippocampal volume and episodic memory performance in preterm children. Dev Neuropsychol. 2013;38(4):226-235. doi: 10.1080/87565641.2013.773003 [DOI] [PubMed] [Google Scholar]
- 46.O'Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64(2):129-155. doi: 10.1016/s0301-0082(00)00054-x [DOI] [PubMed] [Google Scholar]
- 47.Houser CR, Olsen RW, Richards JG, Möhler H. Immunohistochemical localization of benzodiazepine/GABAA receptors in the human hippocampal formation. J Neurosci. 1988;8(4):1370-1383. doi: 10.1523/JNEUROSCI.08-04-01370.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zammit AR, Ezzati A, Zimmerman ME, Lipton RB, Lipton ML, Katz MJ. Roles of hippocampal subfields in verbal and visual episodic memory. Behav Brain Res. 2017;317:157-162. doi: 10.1016/j.bbr.2016.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovachy VN, Adams JN, Tamaresis JS, Feldman HM. Reading abilities in school-aged preterm children: a review and meta-analysis. Dev Med Child Neurol. 2015;57(5):410-419. doi: 10.1111/dmcn.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Families were not consented for data sharing, and for ethical reasons, the data cannot be shared.