Abstract
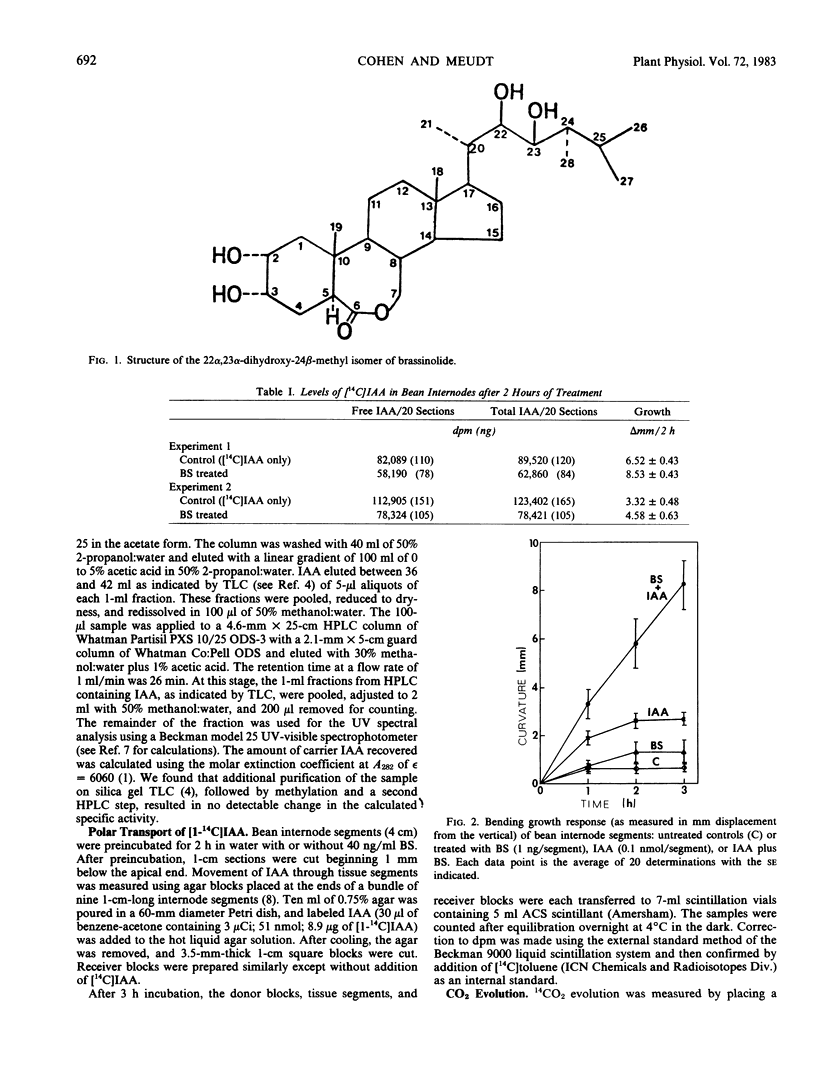
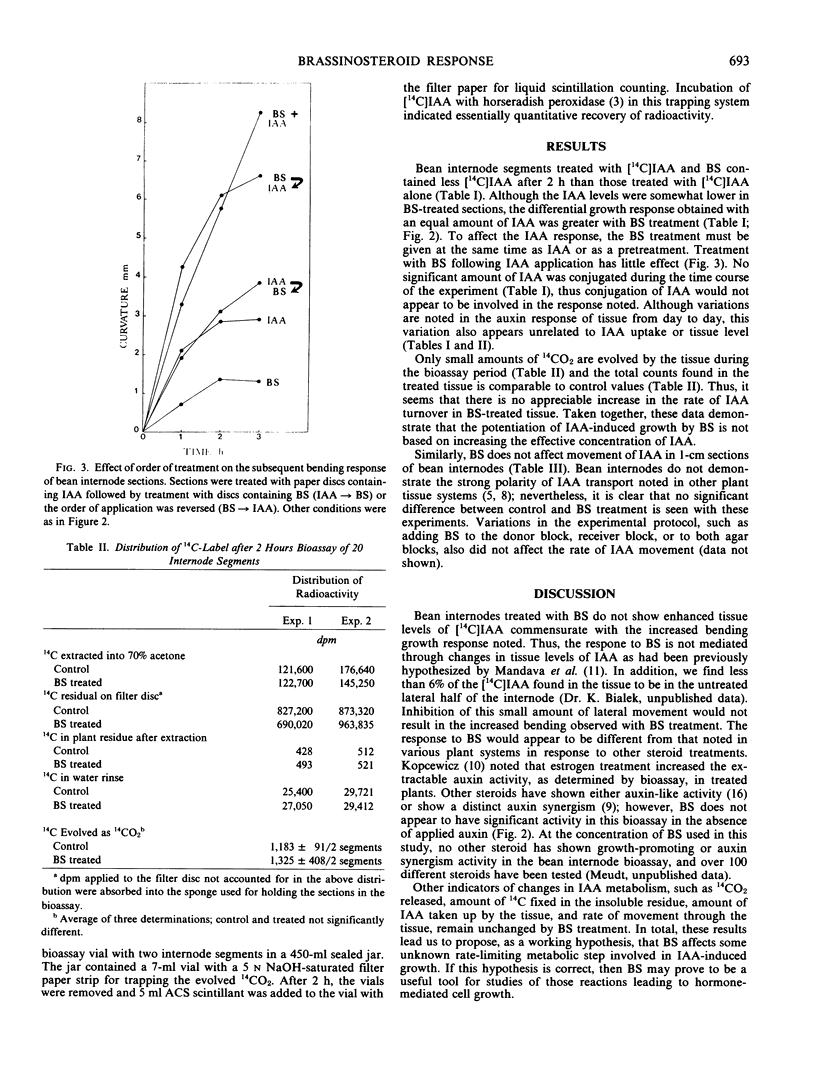
A brassinosteroid treatment of light-grown first internode sections of Phaseolus vulgaris results in an increased bending response following unilateral indole-3-acetic acid (IAA) application. Reverse isotope dilution analysis shows that this increased response is not due to an increase in the concentration of applied IAA in the tissue or a change in the amount of IAA conjugated. Treatment with the brassinosteroid also does not affect the rate of IAA transport as measured using the agar block method. These results indicate that even though brassinosteroid potentiates auxin action, it does not have a direct effect on IAA uptake, metabolism, or cell to cell transport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentrations of Indole-3-acetic Acid and Its Esters in Avena and Zea. Plant Physiol. 1974 Sep;54(3):257–262. doi: 10.1104/pp.54.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Hall P. L., Bandurski R. S. Movement of Indole-3-acetic Acid and Tryptophan-derived Indole-3-acetic Acid from the Endosperm to the Shoot of Zea mays L. Plant Physiol. 1978 Mar;61(3):425–429. doi: 10.1104/pp.61.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter R. P., Peterson M. D., Good N. E. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980 May;65(5):761–767. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcewicz J. Influence of estrogens on the auxins content in plants. Naturwissenschaften. 1970 Jan;57(1):48–48. doi: 10.1007/BF00593574. [DOI] [PubMed] [Google Scholar]
- Mitchell J. W., Mandava N., Worley J. F., Plimmer J. R., Smith M. V. Brassins--a new family of plant hormones from rape pollen. Nature. 1970 Mar 14;225(5237):1065–1066. doi: 10.1038/2251065a0. [DOI] [PubMed] [Google Scholar]
- Thompson M. J., Mandava N. B., Meudt W. J., Lusby W. R., Spaulding D. W. Synthesis and biological activity of brassinolide and its 22 beta, 23 beta-isomer: novel plant growth-promoting steroids. Steroids. 1981 Nov;38(5):567–580. doi: 10.1016/0039-128x(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Thompson M. J., Meudt W. J., Mandava N. B., Dutky S. R., Lusby W. R., Spaulding D. W. Synthesis of brassinosteroids and relationship of structure to plant growth-promoting effects. Steroids. 1982 Jan;39(1):89–105. doi: 10.1016/0039-128x(82)90129-5. [DOI] [PubMed] [Google Scholar]


