Abstract
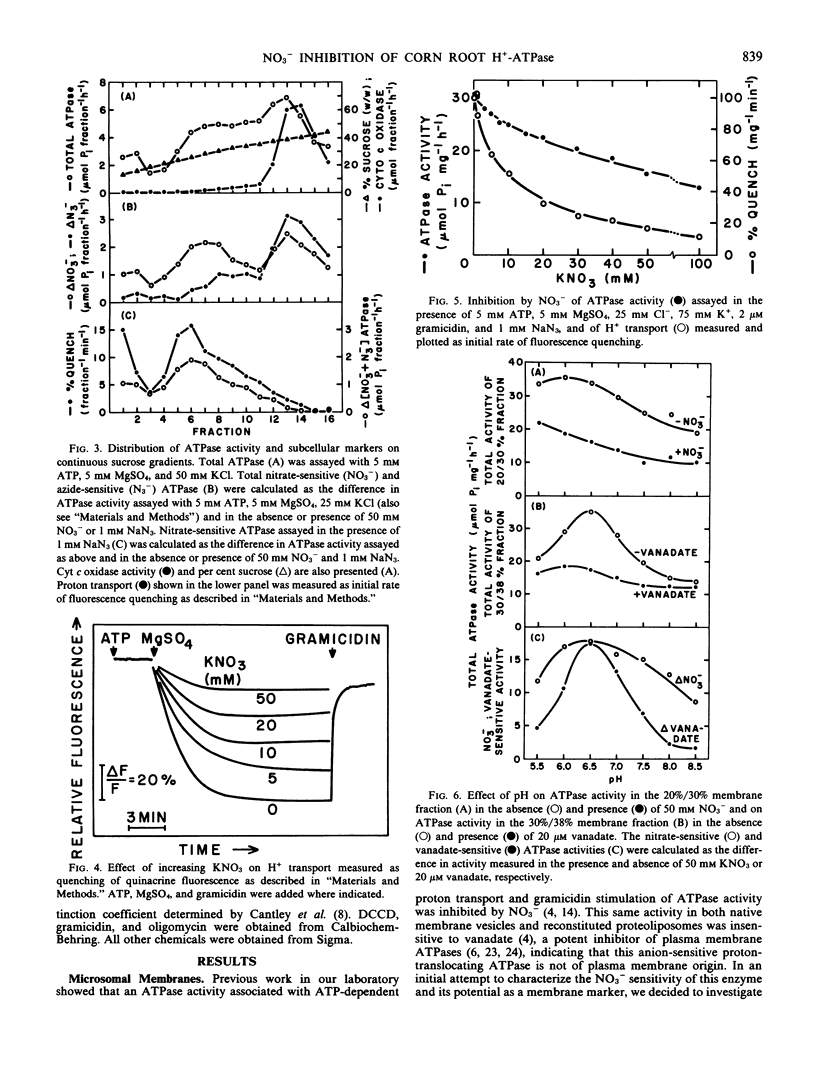
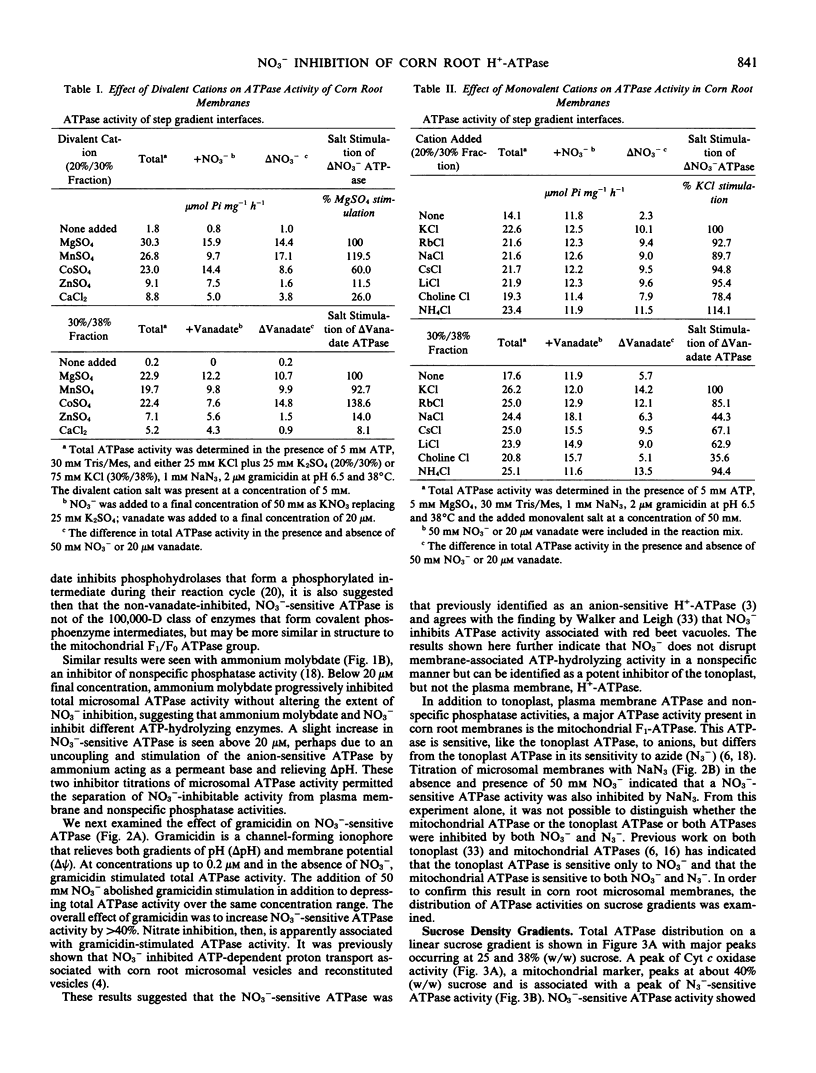
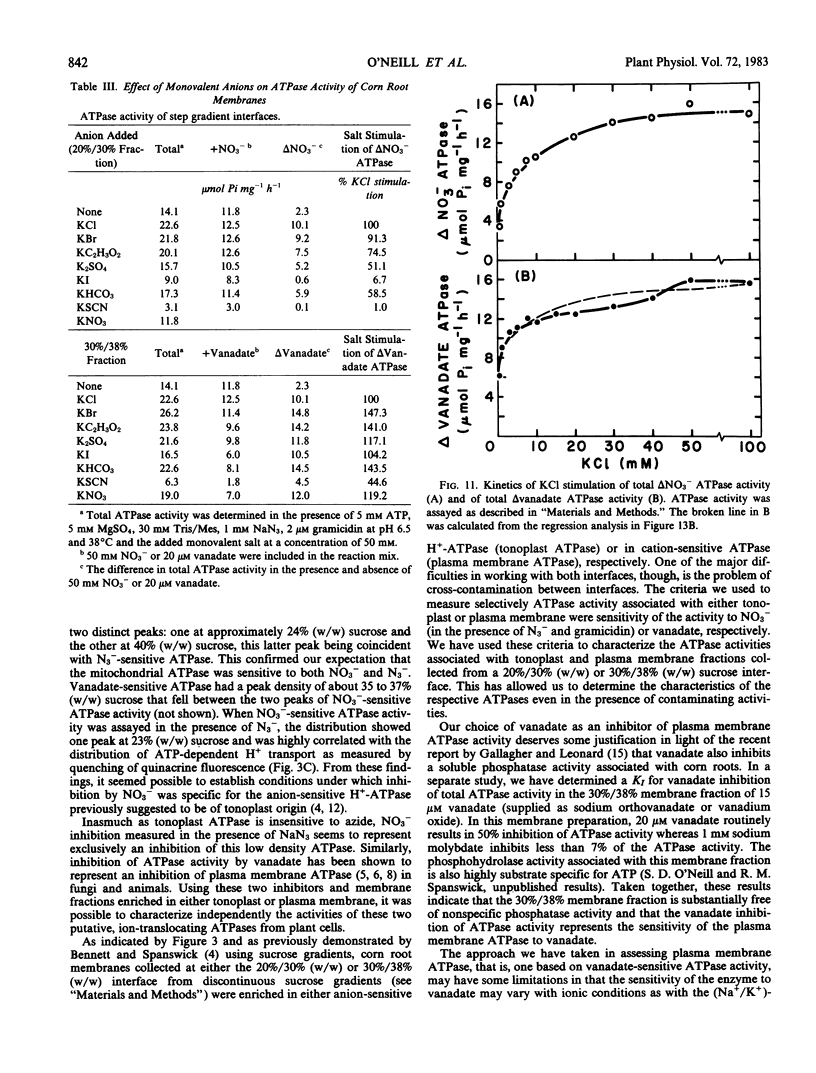
When assayed in the presence of azide, NO3− was shown to be a specific inhibitor of a proton-translocating ATPase present in corn (Zea mays L. cv WF9 × M017) root microsomal membranes. The distribution of the NO3−-sensitive ATPase on sucrose gradients and its general characteristics are similar to those previously reported for the anion-stimulated H+-ATPase of corn roots believed to be of tonoplast origin. An ATPase inhibited by 20 μm vanadate and insensitive to molybdate was also identified in corn root microsomal membranes which could be largely separated from the NO3−-sensitive ATPase on sucrose gradients and is believed to be of plasma membrane origin. Inasmuch as both ATPase most likely catalyze the efflux of H+ from the cytoplasm, our objective was to characterize and compare the properties of both ATPases under identical experimental conditions. The vanadate-sensitive ATPase was stimulated by cations (K+ > NH4+ > Rb+ > Cs+ > Li+ > Na+ > choline+) whereas the NO3−-sensitive ATPase was stimulated by anions (Cl− > Br− > C2H3O2− > SO42− > I− > HCO3− > SCN−). Both ATPases required divalent cations. However, the order of preference for the NO3−-sensitive ATPase (Mn2+ > Mg2+ > Co2+ > Ca2+ > Zn2+) differed from that of the vanadate-sensitive ATPase (Co2+ > Mg2+ > Mn2+ > Zn2+ > Ca2+). The vanadate-sensitive ATPase required higher concentrations of Mg:ATP for full activity than did the NO3−-sensitive ATPase. The kinetics for Mg:ATP were complex for the vanadate-sensitive ATPase, indicating positive cooperativity, but were simple for the NO3−-sensitive ATPase. Both ATPases exhibited similar temperature and pH optima (pH 6.5). The NO3−-sensitive ATPase was stimulated by gramicidin and was associated with NO3−-inhibitable H+ transport measured as quenching of quinacrine fluorescence. It was insensitive to molybdate, azide, and vanadate, but exhibited slight sensitivity to ethyl-3-(3-dimethylaminopropyl carbodiimide) and mersalyl. Overall, these results indicate several properties which distinguish these two ATPases and suggest that under defined conditions NO3−-sensitive ATPase activity may be used as a quantitative marker for those membranes identified tentatively as tonoplast in mixed or nonpurified membrane fractions. We feel that NO3− sensitivity is a better criterion by which to identify this ATPase than either Cl− stimulation or H+ transport because it is less ambiguous. It is also useful in identifying the enzyme following solubilization.
Full text
PDF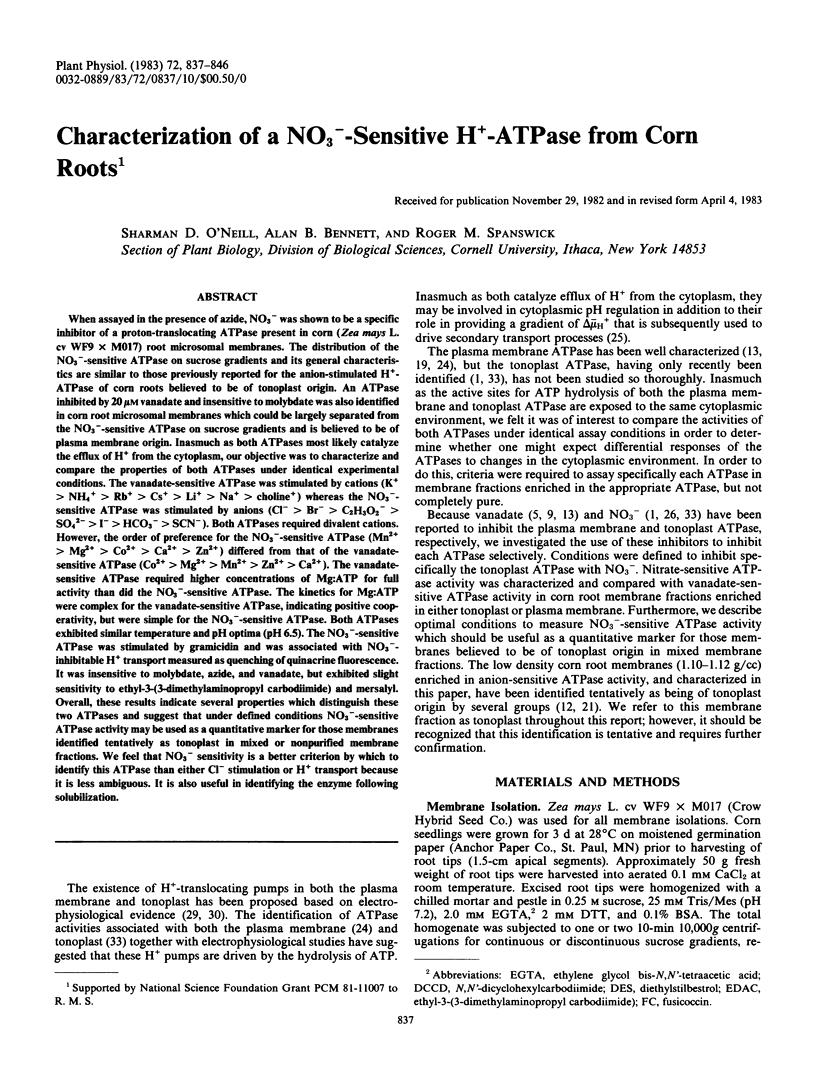





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Burke L. L., Spanswick R. M. Characterization of a partially purified adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1981 Jan;67(1):59–63. doi: 10.1104/pp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Leonard R. T. Solubilization and partial purification of the adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1980 May;65(5):931–938. doi: 10.1104/pp.65.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Characterization of ATPase activity associated with corn leaf plasma membranes. Plant Physiol. 1981 Sep;68(3):521–526. doi: 10.1104/pp.68.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungie J. M., Wiskich J. T. Salt-stimulated Adenosine Triphosphatase from Smooth Microsomes of Turnip. Plant Physiol. 1973 Jun;51(6):1064–1068. doi: 10.1104/pp.51.6.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schoner W., Schmidt H. Inhibition of (Na(+) + K(+))-activated ATPase by N,N'-dicyclohexylcarbodiimide. FEBS Lett. 1969 Nov 29;5(4):285–287. doi: 10.1016/0014-5793(69)80369-8. [DOI] [PubMed] [Google Scholar]
- Sze H. Characterization of nigericin-stimulated ATPase from sealed microsomal vesicles of tobacco callus. Plant Physiol. 1982 Aug;70(2):498–505. doi: 10.1104/pp.70.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]


