Abstract
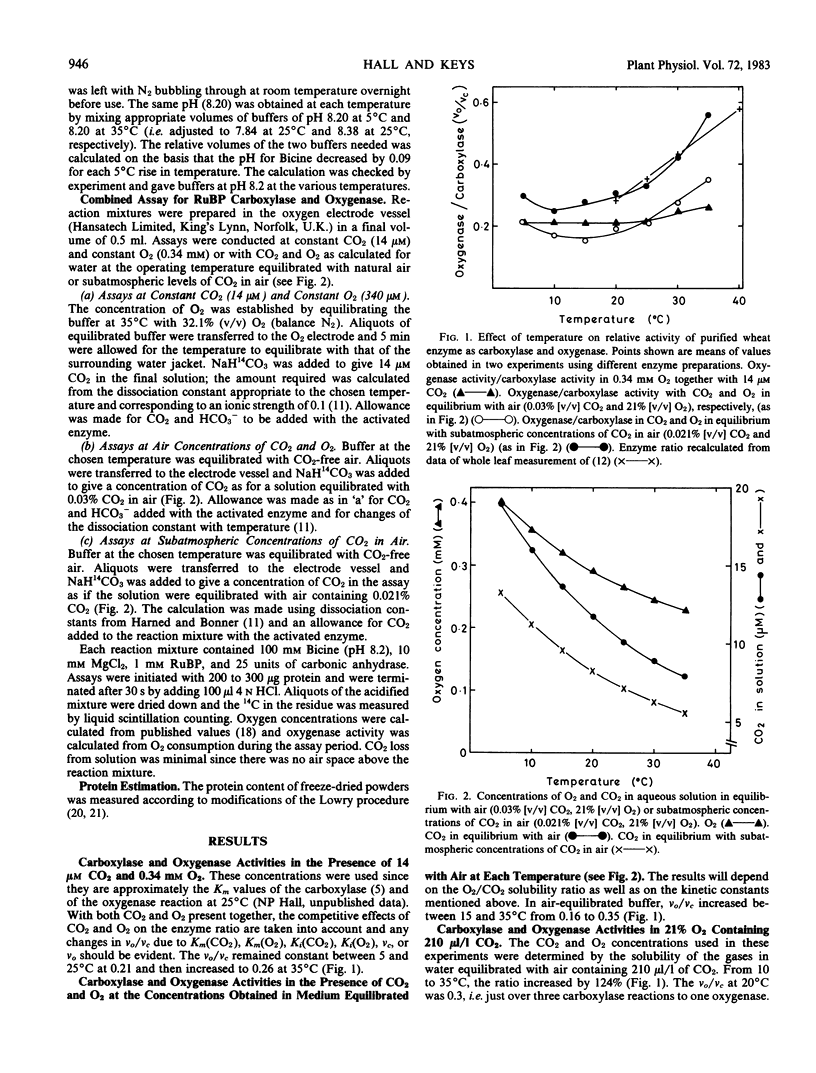
Carboxylase and oxygenase activities of ribulose bisphosphate carboxylase purified from wheat were measured over the temperature range 5 to 35°C either at constant O2 and CO2 concentrations or where the O2 and CO2 simulated the concentrations in water equilibrated at each temperature with the same gaseous phase. At constant CO2 (14 micromolar) and O2 (0.34 millimolar), the oxygenase to carboxylase ratio remained constant at 0.21 between 5 and 25°C but increased to 0.26 at 35°C. At O2 and CO2 concentrations near those expected in water equilibrated with air (21% [v/v] O2) containing 300 μl/l CO2 at the various temperatures, the ratio of oxygenase to carboxylase activity increased 2.2-fold between 15 and 35°C. At CO2 and O2 concentrations expected in water in equilibrium with subatmospheric concentrations of CO2 in air (21% [v/v] O2, 210 μl/l CO2), the oxygenase to carboxylase ratio increased from 0.25 at 10°C to 0.56 at 35°C. Between 20 and 30°C, the apparent Q10 value for the oxygenase reaction was 1.78 and that for the carboxylase was 1.26. Hence, the different responses of photosynthesis and photorespiration to temperature are due more to changes in the relative solubilities of CO2 and O2 (the solubility ratio) than to changes in kinetic parameters of the reactions catalyzed by ribulose bisphosphate carboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Bassham J. A., Krohne S., Lendzian K. In vivo control mechanism of the carboxylation reaction. Basic Life Sci. 1978;11:77–93. doi: 10.1007/978-1-4684-8106-8_6. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Ehleringer J., Björkman O. Quantum Yields for CO(2) Uptake in C(3) and C(4) Plants: Dependence on Temperature, CO(2), and O(2) Concentration. Plant Physiol. 1977 Jan;59(1):86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Parry M. A., Schmidt C. N. The reactions between active and inactive forms of wheat ribulosebisphosphate carboxylase and effectors. Eur J Biochem. 1982 Sep 1;126(3):597–602. doi: 10.1111/j.1432-1033.1982.tb06822.x. [DOI] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Effects of Temperature on Photosynthesis and CO(2) Evolution in Light and Darkness by Green Leaves. Plant Physiol. 1969 May;44(5):671–677. doi: 10.1104/pp.44.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe P. A., Tregunna E. B. Effect of Temperature, CO(2) Concentration, and Light Intensity on Oxygen Inhibition of Photosynthesis in Wheat Leaves. Plant Physiol. 1968 Jun;43(6):902–906. doi: 10.1104/pp.43.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E. Oxygen Inhibition of Photosynthesis: I. Temperature Dependence and Relation to O(2)/CO(2) Solubility Ratio. Plant Physiol. 1977 May;59(5):986–990. doi: 10.1104/pp.59.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Steiger H. M., Beck E. Oxygen Concentration in Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1977 Dec;60(6):903–906. doi: 10.1104/pp.60.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen J. D., Weber J. A., Yocum C. S., Gates D. M. Solubility of gases and the temperature dependency of whole leaf affinities for carbon dioxide and oxygen: an alternative perspective. Plant Physiol. 1979 May;63(5):916–923. doi: 10.1104/pp.63.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Investigation on photorespiration with a sensitive C-assay. Plant Physiol. 1968 Nov;43(11):1829–1837. doi: 10.1104/pp.43.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]


