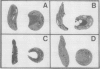
Abstract
The effects of reduced reaction medium osmotic potential (0.67 molar sorbitol as compared to a control treatment with 0.33 molar sorbitol) on the enzymic steps of the photosynthetic carbon reduction cycle were investigated using isolated spinach (Spinacia oleracea L. var Longstanding Bloomsdale) chloroplasts. Reversal of reduced osmotic potential inhibition of photosynthetic rates by a stromal alkalating agent (NH4Cl) was associated with specific steps of the cycle. Low osmotic potential induced stromal acidification was found to be facilitated by osmotically induced chloroplast shrinkage. However, the action of the alkalating agent was found not to be associated with reversal of osmotically induced morphological changes of the stromal compartment.
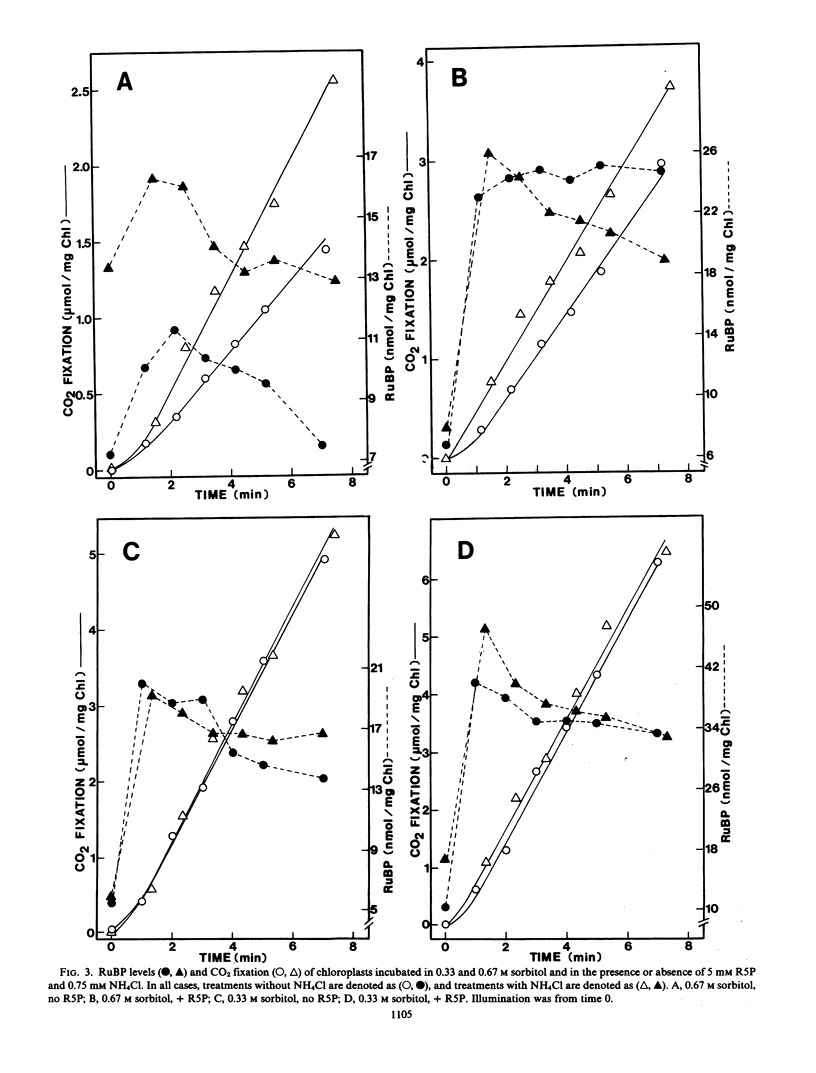
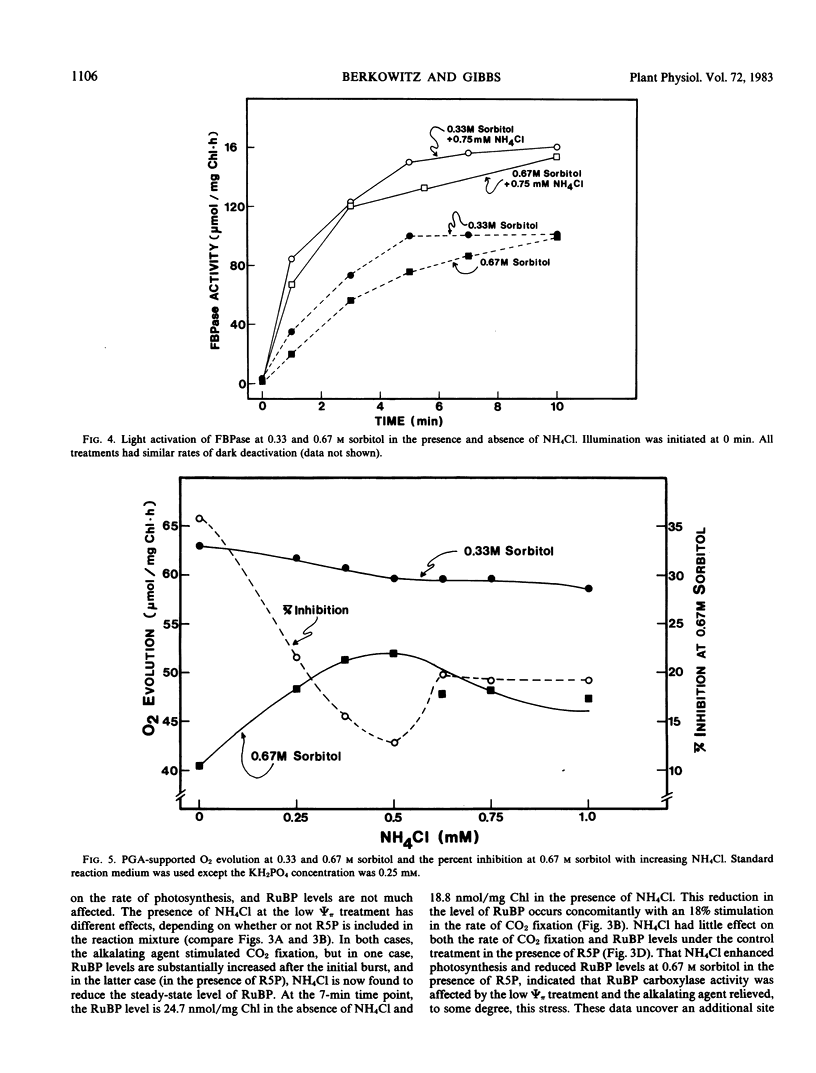
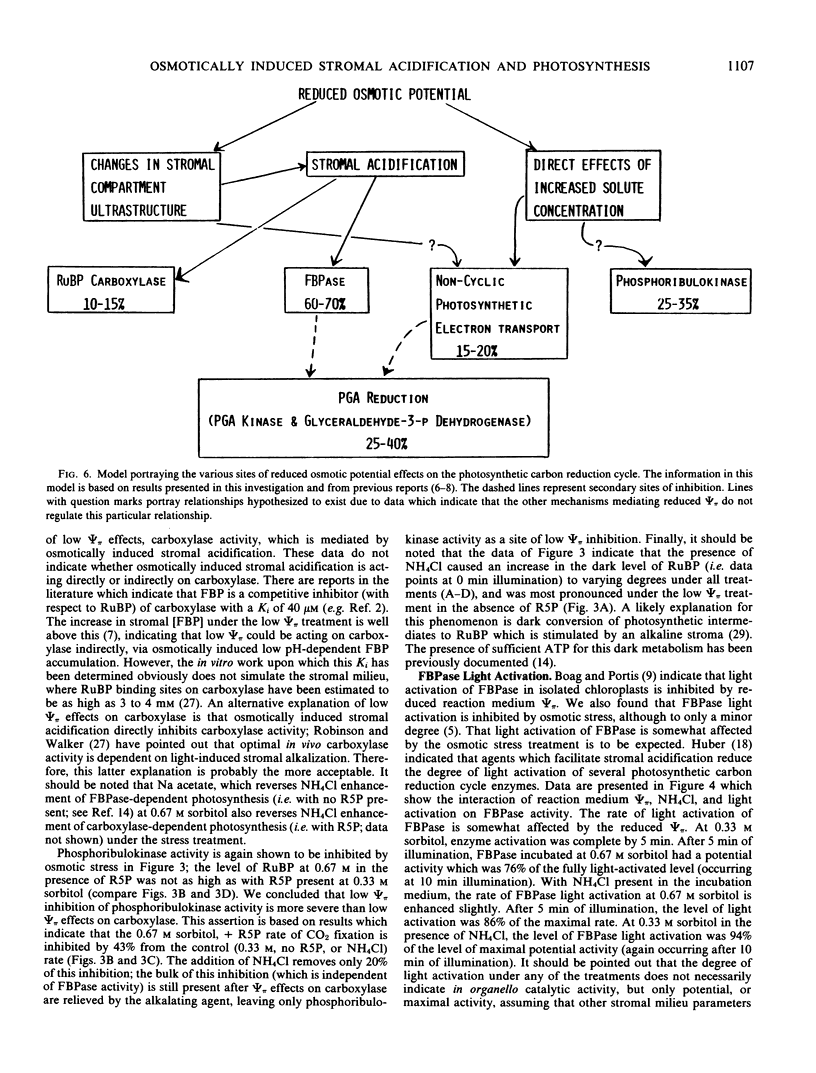
Labeled metabolite analyses indicated that the osmotic stress treatment caused the substrate for fructose 1,6-bisphosphatase (FBPase) to build up in the absence of NH4Cl, and the substrate for phosphoribulokinase to increase in the presence of NH4Cl. These data were interpreted as indicating that the most severe effect of osmotic stress on photosynthesis is at the site of FBPase, and that this inhibition is mediated by osmotically induced stromal acidification. Phosphoribulokinase activity inhibition at the low osmotic potential treatment was apparently less severe and not mediated by stromal acidification. A third site of osmotic inhibition, which was reversed by NH4Cl, and therefore was assumed to be mediated by stromal acidification, was at the step of ribulose 1,5-bisphosphate carboxylase.
Additions of NH4Cl also enhanced the activity of the pH-insensitive phase of the photosynthetic carbon reduction cycle, 3-phosphoglyceric acid reduction, at the stress treatment. This effect was thought to be mediated by the removal of the block at FBPase. A model was proposed to outline the relative severity of osmotic stress effects at various sites of the photosynthetic carbon reduction cycle.
Full text
PDF

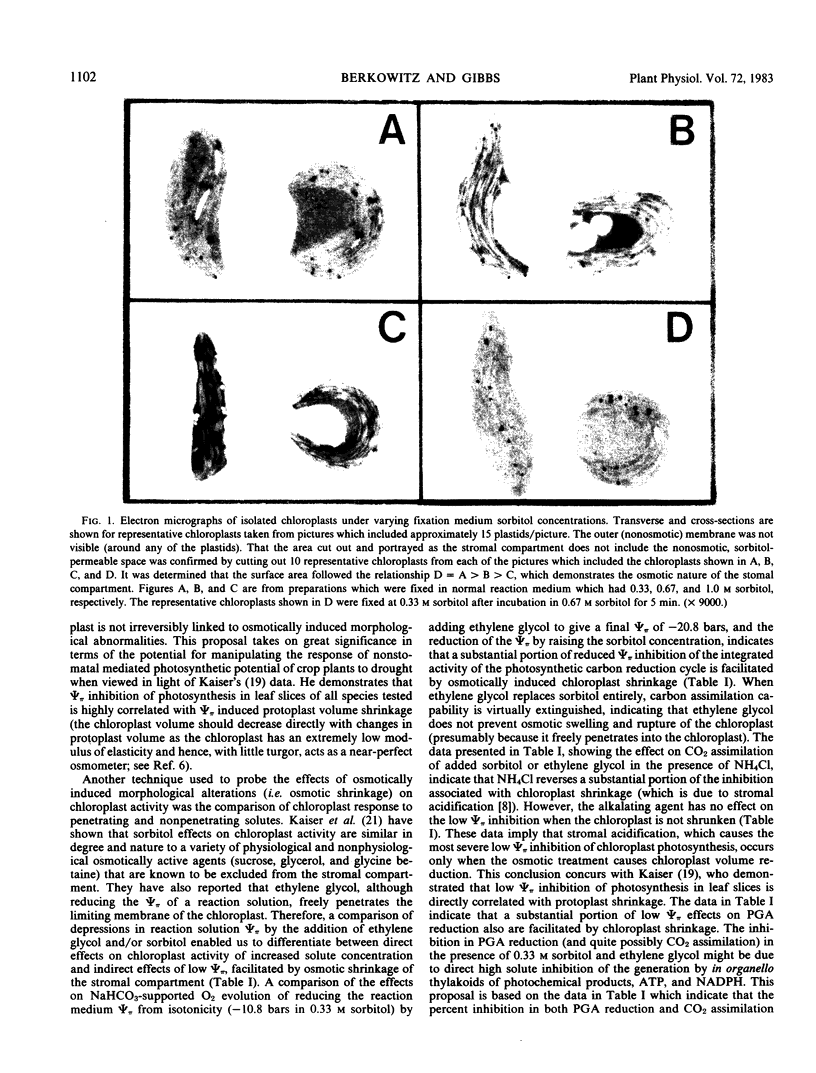
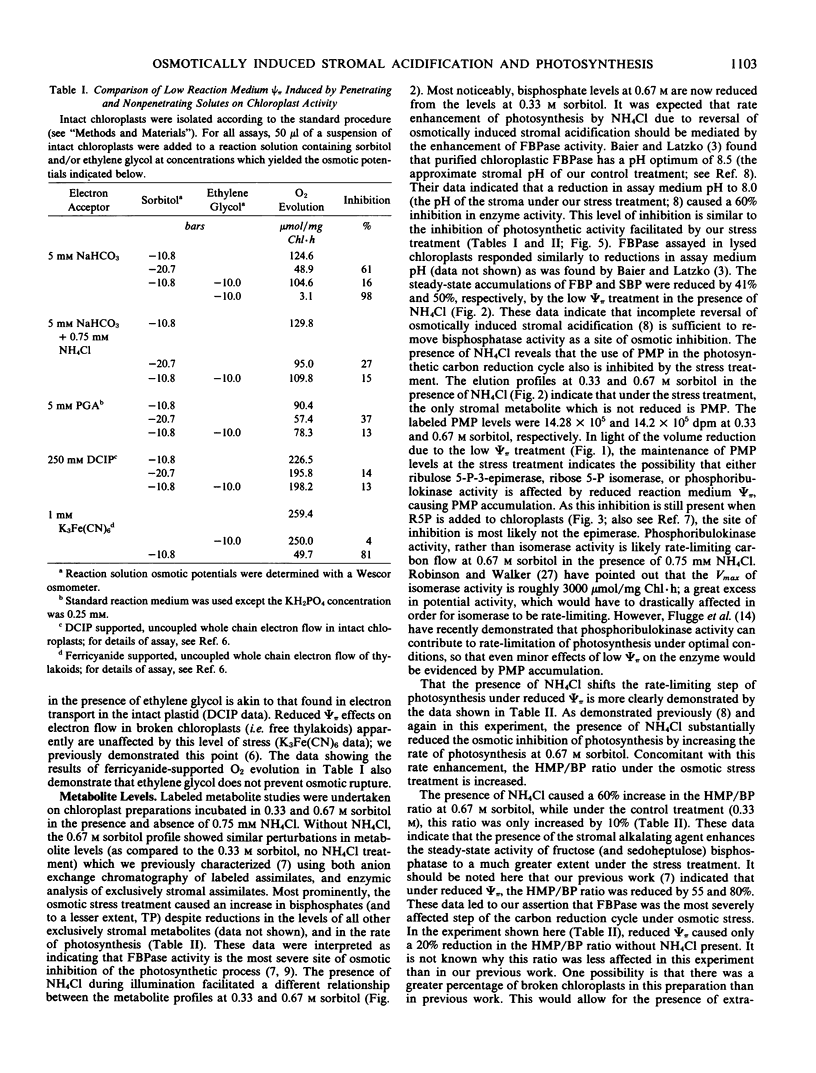
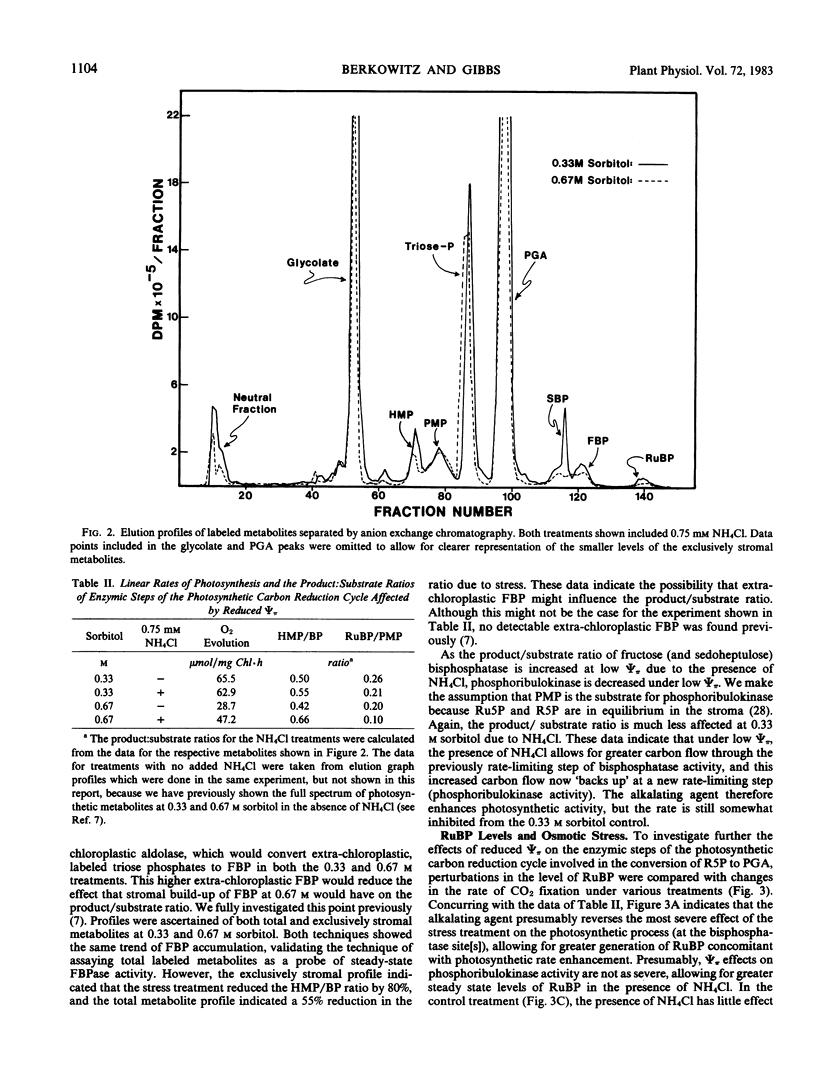


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Lorimer G. H. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981 Apr 14;20(8):2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Effect of osmotic stress on photosynthesis studied with the isolated spinach chloroplast : generation and use of reducing power. Plant Physiol. 1982 Oct;70(4):1143–1148. doi: 10.1104/pp.70.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Effect of osmotic stress on photosynthesis studied with the isolated spinach chloroplast : site-specific inhibition of the photosynthetic carbon reduction cycle. Plant Physiol. 1982 Nov;70(5):1535–1540. doi: 10.1104/pp.70.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen M. E., Freeman T. P. Partial Restoration of the High Rate of Plastid Pigment Development and the Ultrastructure of Plastids in Detached Water-stressed Wheat Leaves. Plant Physiol. 1975 Apr;55(4):768–773. doi: 10.1104/pp.55.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Stitt M., Freisl M., Heldt H. W. On the Participation of Phosphoribulokinase in the Light Regulation of CO(2) Fixation. Plant Physiol. 1982 Jan;69(1):263–267. doi: 10.1104/pp.69.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Regulation of chloroplast photosynthetic activity by exogenous magnesium. Plant Physiol. 1978 Sep;62(3):321–325. doi: 10.1104/pp.62.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut Z. Inhibition of photosynthetic carbon dioxide fixation in isolated spinach chloroplasts exposed to reduced osmotic potentials. Plant Physiol. 1971 Nov;48(5):591–595. doi: 10.1104/pp.48.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Sicher R. C., Bahr J. T., Jensen R. G. Measurement of ribulose 1,5-bisphosphate from spinach chloroplasts. Plant Physiol. 1979 Nov;64(5):876–879. doi: 10.1104/pp.64.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]