Abstract
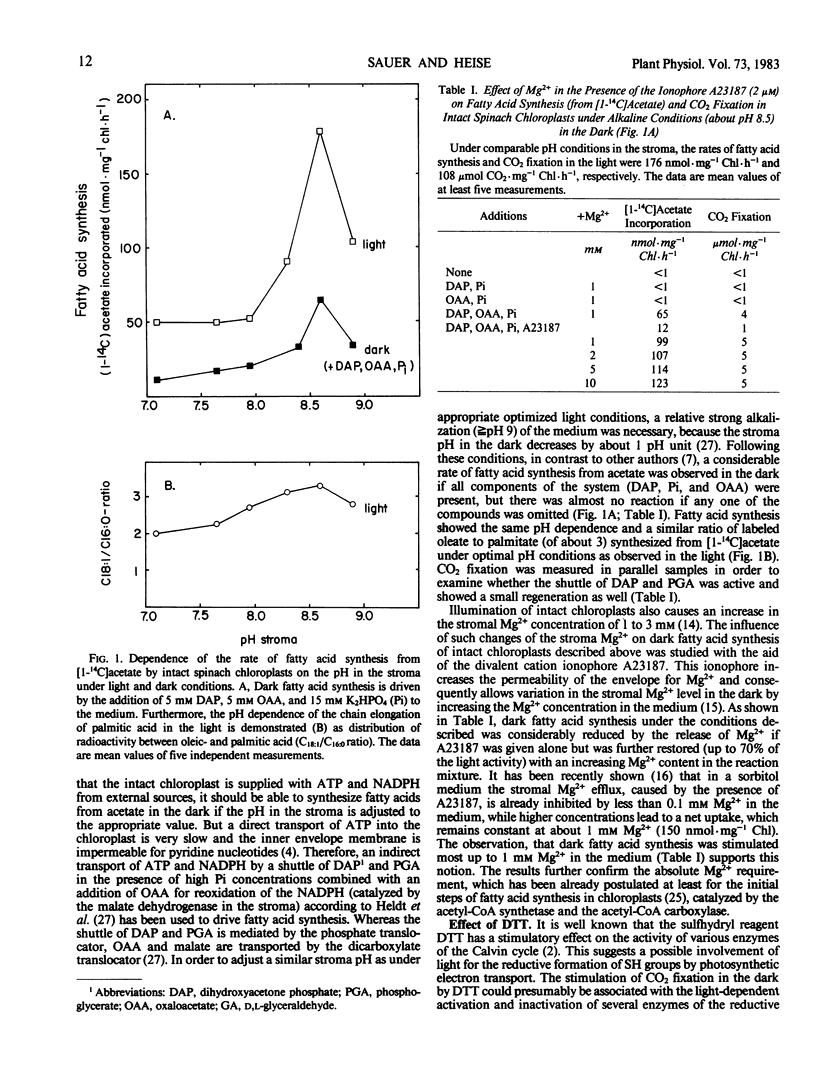
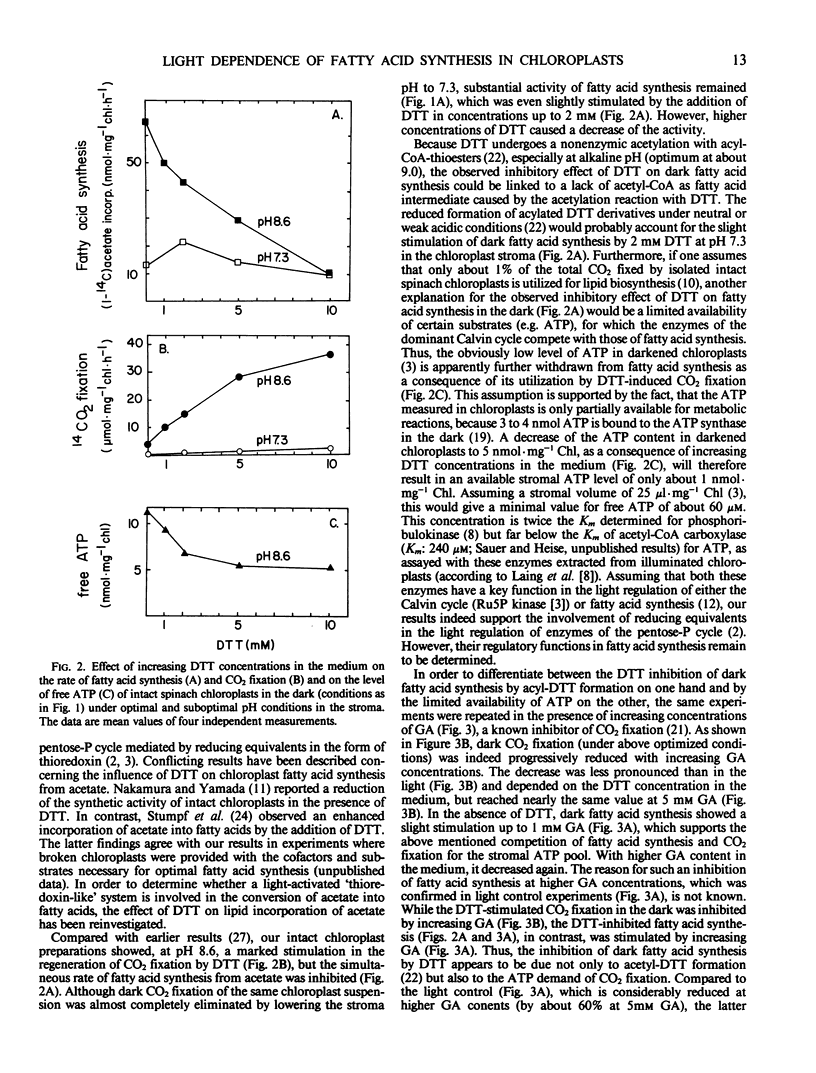
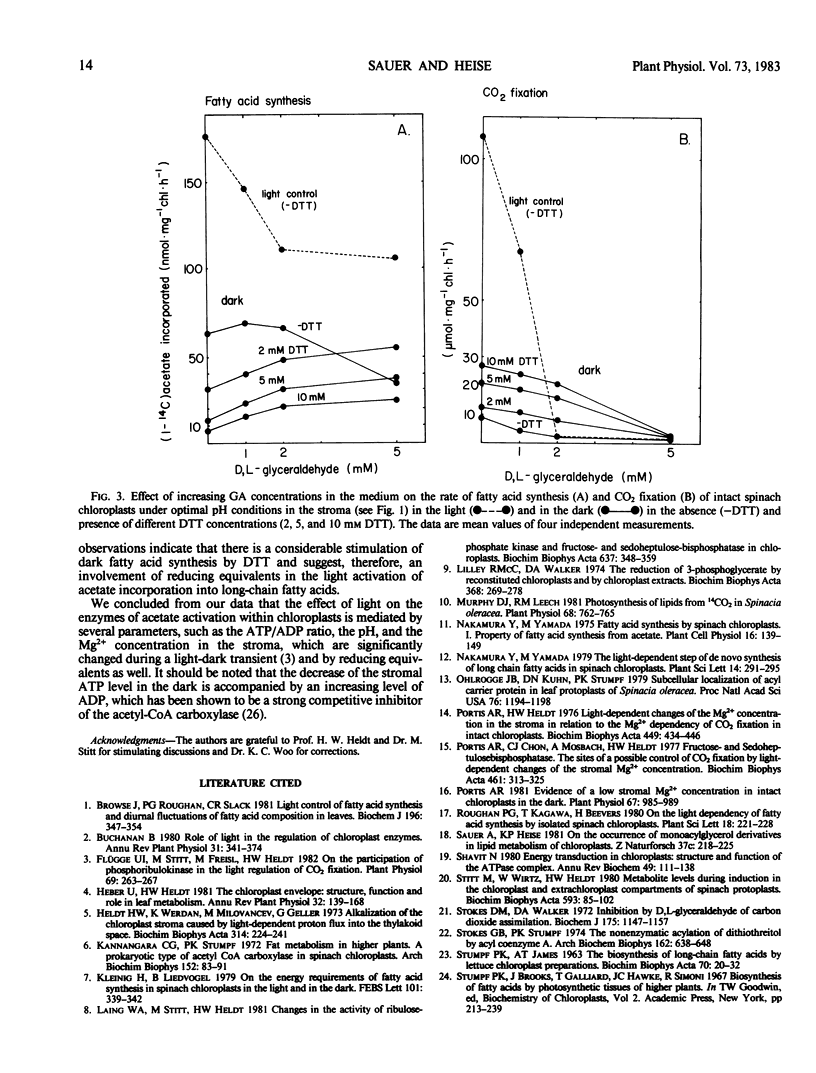
The capacity of intact chloroplasts to synthesize long chain fatty acids from acetate depends on the stroma pH in Spinacia oleracea, U. S. hybrid 424. The pH optimum is close to 8.5. Lowering of the stroma pH leads to a reduction of acetate incorporation but does not suffice to eliminate fatty acid synthesis completely. Chain elongation from palmitic to oleic acid shows the same pH dependence. Fatty acid synthesis is activated in the dark upon the simultaneous addition of dihydroxyacetone phosphate and orthophosphate supplying ATP and oxaloacetate for reoxidation of NADPH in the stroma. Under these conditions both dark fatty acid synthesis and synthesis of oleate from palmitate show the same pH dependence as in the light. Dark fatty acid synthesis is further stimulated by increasing the stromal Mg2+ concentration with the ionophore A 23187. In contrast to CO2 fixation, dark fatty acid synthesis is considerably reduced by dithiothreitol (DTT). This observation may be due to an acetyl-CoA deficiency, caused by a nonenzymic acylation of DTT, and a competition for ATP between DTT-activated CO2 fixation and fatty acid synthesis. Because d,l-glyceraldehyde as inhibitor of CO2 fixation compensates the DTT effect on dark fatty acid synthesis, reducing equivalents may be involved in the light dependence of acetate activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browse J., Roughan P. G., Slack C. R. Light control of fatty acid synthesis and diurnal fluctuations of fatty acid composition in leaves. Biochem J. 1981 Apr 15;196(1):347–354. doi: 10.1042/bj1960347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Stitt M., Freisl M., Heldt H. W. On the Participation of Phosphoribulokinase in the Light Regulation of CO(2) Fixation. Plant Physiol. 1982 Jan;69(1):263–267. doi: 10.1104/pp.69.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. On the energy requirements of fatty acid synthesis in spinach chloroplasts in the light and in the dark. FEBS Lett. 1979 May 15;101(2):339–342. doi: 10.1016/0014-5793(79)81039-x. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Leech R. M. Photosynthesis of Lipids from CO(2) in Spinacia oleracea. Plant Physiol. 1981 Sep;68(3):762–765. doi: 10.1104/pp.68.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R. Evidence of a Low Stromal Mg Concentration in Intact Chloroplasts in the Dark: I. STUDIES WITH THE IONOPHORE A23187. Plant Physiol. 1981 May;67(5):985–989. doi: 10.1104/pp.67.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Chon C. J., Mosbach A., Heldt H. W. Fructose-and sedoheptulosebisphosphatase. The sites of a possible control of CO2 fixation by lightdependent changes of the stromal Mg2+ concentration. Biochim Biophys Acta. 1977 Aug 10;461(2):313–325. doi: 10.1016/0005-2728(77)90181-5. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]
- Shavit N. Energy transduction in chloroplasts: structure and function of the ATPase complex. Annu Rev Biochem. 1980;49:111–138. doi: 10.1146/annurev.bi.49.070180.000551. [DOI] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The nonenzymatic acylation of dithiothreitol by acyl coenzyme A. Arch Biochem Biophys. 1974 Jun;162(2):638–648. doi: 10.1016/0003-9861(74)90226-4. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


