Abstract
This two-paper Series focuses on recent advances and applications of regenerative medicine that could benefit paediatric patients. Innovations in genomic, stem-cell, and tissue-based technologies have created progress in disease modelling and new therapies for congenital and incurable paediatric diseases. Prenatal approaches present unique opportunities associated with substantial biotechnical, medical, and ethical obstacles. Maternal plasma fetal DNA analysis is increasingly adopted as a noninvasive prenatal screening or diagnostic test for chromosomal and monogenic disorders. The molecular basis for cell-free DNA detection stimulated the development of circulating tumour DNA testing for adult cancers. In-utero stem-cell, gene, gene-modified cell (and to a lesser extent, tissue-based) therapies have shown early clinical promise in a wide range of paediatric disorders. Fetal cells for postnatal treatment and artificial placenta for ex-utero fetal therapies are new frontiers in this exciting field.
Introduction
Advances in stem-cell biology and tissue engineering technologies are ideally suited to treat congenital diseases that do not have alternative therapeutic options.1 An example of a paediatric application of regenerative medicine technology is the use of patient-specific organoids derived from rectal biopsies of patients with cystic fibrosis, to quantitate individual drug response in vitro, thereby allowing the prospective choice of more efficacious treatments for the patient.2 Laboratory discoveries for regenerative medicine hold great promise; however, clinical translation remains a major challenge. Understanding disease modelling and novel gene, cell, and tissue-based therapies of rare congenital diseases (and any subsequent progression) can advance the entire field of regenerative medicine with first-in-human breakthroughs. There are also areas in which paediatric regenerative medicine is leading the way, with approaches in adult regenerative medicine closely following its progress. One example is noninvasive prenatal testing by analysis of fetal DNA in maternal plasma, which has helped the development of liquid diagnosis for adult cancers.3,4 Examples of clinical trials of regenerative medicine for paediatric diseases and advances in the field will be discussed in this Series.
Technological platforms
Stem and progenitor cells
Pluripotent and multipotent stem cells with the capacity to self-renew and differentiate into target cell types are a cornerstone of regenerative medicine. Stem-cell therapy represents the most established treatment modalities within regenerative medicine. The first successful haematopoietic cell transplantations were reported in the late 1950s, and in the following 60 years, advances in the understanding of immune modulation have drastically improved outcomes for patients, with current survival exceeding 90% in cases of nonmalignant haematological disorders. Haematopoietic stem and progenitor cells (HSCs) were the first cell type successfully used in a fetal setting to cure patients with immunodeficiencies, and they form the focus of several recent high-profile trials of autologous ex-vivo gene-correction for haemoglobinopathies.5,6 Additionally, mesenchymal stem and progenitor cells (MSCs) are multipotent cells that have the potential to differentiate into the osteogenic, chondrogenic, myogenic, and adipogenic lineages, and they have a minimal oncogenic risk. MSCs display a non-immunogenic profile, allowing transplantation across major histocompatibility barriers without immunosuppression. Due to these favourable characteristics, MSCs are tested in clinical trials for many disorders.7 Finally, advances in embryonic stem-cell biology have been pivotal in the field, but the greatest clinical effect has arisen from the success of human-induced pluripotent stem-cell (iPSC) applications for disease modelling and new therapeutics.8 Although it is unlikely that iPSCs will be transplanted directly to the fetus, targeted differentiation to multipotent stem and progenitor cells (such as HSCs and MSCs) could extend their utility to prenatal therapy. Stem cells (eg, embryonic, iPSC, or adult organ-specific) can be grown into self-organising three dimensional (3D) organoids in vitro; for example, human fetal hepatocytes have been used successfully to overcome difficulties of expansion of primary human hepatocytes.9 However, organoid technologies are generally more applicable for postnatal applications.85
Genetic engineering
Genetic engineering is a broad term encompassing gene addition, knockdown, and, more recently, editing. The vectors delivering these genetic engineering platforms are diverse—they can be separated into non-viral vectors and engineered viruses, and might integrate into the genome (such as lentiviruses) or exist outside the nucleus (such as adeno-associated viruses [AAV]).10 The Nobel prize winning discovery of CRISPR–Cas9 technology by Charpentier and Doudna as a precise gene-editing tool has provided unprecedented opportunities to improve understanding and to cure both genetic and nongenetic diseases.11 The ability to precisely nick and repair or replace a defective genetic sequence opens a therapeutic window for thousands of monogenic diseases. Cells or tissues might also be altered, generated, or regenerated specifically by manipulation and engineering of the cell genome (either ex vivo or in situ) to restore typical physiology for patients with nongenetic diseases.
Ongoing concerns persist around safety (specifically immunogenicity of transgenes and vectors and the potential for off-target effects) and efficacy (specifically non-specific targeting and inefficient transduction or an inadequate durable effect). Technologies addressing many of these concerns are currently being researched extensively in various preclinical models, and have been reviewed extensively. Although a viral vector or ex-vivo approach will probably represent the first application of gene therapy to a fetus, it is probable that nonviral vectors and in-vivo genetic editing will represent the prevailing future direction of the field.12
Cell signalling manipulation
During fetal development, morphogenesis is hierarchical and involves various stem and progenitor cells that are tightly regulated spatially and temporally by paracrine mediators. To recapitulate this highly complex developmental process for regenerative medicine, a cell-based, cell-free, or combined therapeutic approach is often used. Paracrine factors can be directly introduced as small molecules, recombinant proteins, synthetic modified mRNA, small non-coding RNA (such as microRNA), antisense oligonucleotides, or extracellular vesicles (such as exosomes).13 A recent example is the use of cardiosphere-derived exosomal microRNA for myocardial repair in paediatric dilated cardiomyopathy.14 Following encouraging preclinical investigations, a prospective phase 1a study was conducted in five patients and showed safety and improved cardiac function, thus laying the foundation for further randomised trials.
Delivery of therapy to the fetus
Delivery of any treatment in utero is particularly challenging, considering that regenerative medicine-based therapies need early gestation delivery. Ultrasonic tracking systems have been developed for accurate identification of the needle tip, and multimodal navigation systems can combine detailed prenatal imaging with accurate image-guided instrumentation.15 Multimodal navigation systems can provide clear advantages for some prenatal interventions, and have been tested in fetoscopic laser photocoagulation for the treatment of twin-to-twin transfusion syndrome. Similar to other in-utero interventions, this procedure is particularly challenging due to the limited field of view, poor visibility, and poor image quality. Fetoscopic mosaicking can help to create an image with an expanded field of view, which could aid clinicians when performing minimally-invasive fetal interventions.16 Moreover, the development of smaller instruments will help to decrease the risks of prenatal intervention to the pregnancy and will allow the delivery of less invasive, tailormade, regenerative medicine-based therapies to the fetus, for example, single-access fetal endoscopy for the management of myelomeningocele (MMC), which has been tested successfully in sheep.17 Ultimately, robot-assisted technology coupled with artificial intelligence and machine learning could address some of the challenges related to early fetal intervention. Such platforms could augment accuracy and dexterity, enhance efficacy and safety, and ultimately improve outcomes of fetal intervention.18
Indications, limitations, and ethical considerations of fetal therapy
The unique fetal physiology provides multiple advantages for treating congenital diseases prenatally. The average weight of a 20-week-old fetus is over ten times lower than an average term birthweight, allowing a high cell or viral particle number-to-weight dosage compared with treating the neonate, which results in better efficacy and a cost advantage.8 The foramen ovale and the ductus arteriosus permit systemic infusions into the umbilical vein to bypass the fetal lungs, avoiding cell sequestration in the lung microvasculature, which occurs in postnatal infusion. Cell proliferation and migration to different anatomical compartments allows for wider engraftment of donor cells and facilitates integration of therapeutic transgenes delivered through viral vectors or gene-editing technologies. Donor-specific immune tolerance might be facilitated, as during fetal life the immune system undergoes self-education, and at specific transplantation times, foreign cells might be recognised as self. Not only does this recognition permit acceptance of the graft without myeloablation or immunosuppression, which is required for postnatal transplantation, it might also enable postnatal booster transplantation with the same donor cells. Psychologically, in-utero treatment might also offer an advantage for prospective parents of an affected fetus, as instead of the only options being terminating the pregnancy or awaiting the delivery of a severely affected child, there is the prospect of an active fetal treatment and potential cure.
However, safely introducing fetal therapy into practice has several challenges, not least the emotive environment in which parents and health-care practitioners will need to make rapid judgements and decisions. Involving patient groups and parents with experience of the condition to be treated can overcome potential ethical hurdles of when to approach trial participation, inclusion and exclusion criteria, and primary outcome measures.19,20 The International Fetal Transplantation and Immunology Society (IFeTIS) recently facilitated a panel discussion to define best practice and to consider safety aspects, patient monitoring, and managing ethical dilemmas.21 Safety evaluations must consider the risks of both the mode of administration and the product itself to the fetus and to the mother. Risks include fetal bleeding and loss of the fetus, although large case series of fetal blood transfusion for anaemia provide reassurance that minimally invasive, ultrasound-guided injection into the umbilical vein is safe.22 Fetal interventions might need delivering in the first trimester to avoid a competent fetal immune response. Techniques such as intracardiac injection have been evaluated in non-human primates with some success.23 Adverse events are most likely to occur in the short term after fetal therapy, and can now be defined and graded using the first systematic Maternal and Fetal Adverse Event Terminology.24 Fetal monitoring remains a challenge, particularly before 32 weeks when interpretations of cardiotocography are compromised by the physiological immaturity of the cardiovascular and neurological systems.25 The decision to perform an emergency caesarean section in the event of a life-threatening fetal complication will need careful discussion between the parents and health-care providers, taking into consideration the potential quality of life at the gestational age of the intervention.
Noninvasive prenatal testing: fetal genome, epigenome, and transcriptome in maternal plasma
Noninvasive prenatal testing with massively parallel sequencing of cell-free fetal DNA in maternal plasma has enabled high-throughput deep sequencing and analysis of fetal genome, from chromosomal abnormalities to single-gene disorders.26 In the clinical setting, noninvasive prenatal testing is currently used to screen for trisomy 21 and aneuploidies, particularly in ultrasound screenpositive pregnancies such as those with increased nuchal translucency. The implementation of noninvasive prenatal testing for chromosomal aneuploidies has led to a reduction in invasive testing performed for prenatal diagnosis. Noninvasive prenatal testing is being applied clinically to diagnose single-gene disorders such as fibroblast growth factor receptor mutations associated with skeletal dysplasias, β-thalassaemia, and for fetal rhesus blood group D antigen genotypes in RhD-negative pregnant people.3,27 Noninvasive prenatal testing for multiple Mendelian monogenic disorders has been reported with high accuracy.4 However, it must be noted that the small amount of fetal DNA during early pregnancy and the high maternal BMI might give rise to false-negative results. In addition to DNA sequence variations, epigenetic changes are also implicated in gene regulation and fetal development, with DNA methylation being one of the best known epigenetic modifications. By identifying and studying placenta-specific methylation markers serially, any methylation status change might help with monitoring obstetric disease such as intrauterine growth restriction.28
In addition to DNA, fetal RNAs are also released into the mother’s bloodstream. The measurement of fetal RNAs might reflect changes in the fetoplacental transcriptome and offer an insight into alterations in placental function, which could then be used to detect fetal hypoxia and to predict obstetric disease such as preeclampsia.29
With further advances in whole-genome haplotypephasing techniques and newer sequencing platforms, it is envisioned that fetal genomic, transcriptomic, and methylomic analysis could become part of routine prenatal care, providing more clinically meaningful data on disease severity, prevalence, and prognosis. Nonetheless, for many conditions, expectant parents should be clearly informed that noninvasive prenatal testing is not diagnostic, and that any high-risk result should be confirmed with invasive testing such as amniocentesis or chorionic villus sampling, supported by the appropriate counselling.
In-utero stem-cell therapy
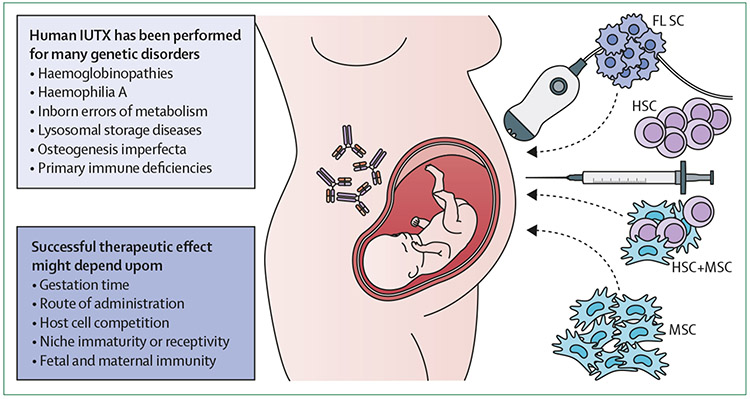
Preclinical studies in small and large animal models have shown the feasibility of using in-utero stem-cell transplantation to correct a wide variety of genetic disorders. Studies of in-utero stem-cell transplantation of HSC showed that successful chimerism was achieved, but that gestation days, route of administration, host cell competition, niche immaturity, and fetal and maternal immunity limited engraftment, thereby reducing donor-derived haematopoiesis (figure 1).30 In the mid 1990s, attempts to increase rates of HSC engraftment by cotransplanting MSCs indicated that MSCs engrafted and promoted HSC differentiation after in-utero stem-cell transplantation. Successful fetal-derived MSC engraftment in bone after in-utero stem-cell transplantation in a patient with severe osteogenesis imperfecta established the ability of MSCs to integrate and differentiate into bone. However, the use of MSCs in utero in general has provided little evidence of clinically meaningful engraftment, and most effects appear to be paracrine.
Figure 1: Prenatal stem-cell therapy.
IUTX has been performed in several disorders using different types and sources of cells. Successful therapeutic effect might depend upon the gestational age, the route of administration, host cell competition, niche receptivity, and fetal and maternal immunity (such as the presence of maternal antibodies crossing the placenta, depicted as the antibodies on the mother and fetus). IUTX=in-utero stem-cell transplantation. HSC=haematopoietic stem and progenitor cell. MSC=mesenchymal stem and progenitor cell. FLSC=fetal liver stem cell.
Human in-utero stem-cell transplantation has been performed on 46 fetal recipients for 14 different genetic disorders, including primary immune deficiencies, haemoglobinopathies, inborn errors of metabolism, lysosomal storage diseases, and haemophilia A (reviewed by Ekblad-Nordberg and colleagues;32 figure 1 and table). Unfortunately, these studies showed that in-utero stem-cell transplantation was not able to establish clinically relevant rates of HSC engraftment, except in primary immune deficiencies. This result is due to the fact that these attempts have used access methods during early gestation that were inefficient (such as intraperitoneal injection), or the methods were performed too late in gestation for success (see the immunological considerations section).33
Table.
Summary of all known published cases on prenatal transplantation of fetal cells (not including blood transfusions performed in utero) for various diseases
| Disease | Cells administered |
Fetal site for administration |
Gestational age (weeks) |
Cell dose | Number of prenatal doses |
Complications at procedure |
Maternal complications |
|
|---|---|---|---|---|---|---|---|---|
| Westgren et al (1996) | α-thalassaemia | Fetal liver cells | Umbilical vein | 15, 31 | 20·4 × 108 per kg and 1·2 × 108 cells per kg | 2 | Uncomplicated | No data |
| Cowan et al (1994) | β-thalassaemia | Fetal liver cells | Umbilical vein | ·· | ·· | 1 | Septic miscarriage | No comment |
| Orlandi et al (1996) | β-thalassaemia | Fetal blood cells | Umbilical vein | 19 | 0·8 mL fetal blood | 1 | Uncomplicated | No comment |
| Westgren et al (1996) | β-thalassaemia | Fetal liver cells | Umbilical vein | 18 | 8·6 × 108 cells per kg | 1 | Uncomplicated | The pregnancy continued uneventfully |
| Touraine et al (2004) | β-thalassaemia | Fetal liver and thymic cells | Intraperitoneally | 14 | 3 × 108 cells per kg | 1 | Uncomplicated | No adverse events in the mother |
| Touraine et al (2004) | β-thalassemia | Fetal liver and thymic cells | Umbilical vein | 19 | ·· | 1 | Bradycardia and fetal death | No comment |
| Touraine et al (2004) | Bare lymphocyte syndrome | Fetal liver and thymus cells | Umbilical vein | 30 | 1·6 × 107 cells per fetus | 1 | Uncomplicated | No comment |
| Touraine et al (2004) | Chronic granulomatous disease | Fetal liver cells | Umbilical vein | 19, 23 | ·· | 2 | Bradycardia and fetal death | No comment |
| Steven Shaw, Chang Gung Memorial Hospital, Linkou, Taiwan | Gaucher disease type 2 | Fetal MSC | Umbilical vein | 32 | 5·5 × 106 cells per kg | 1 (+ 6 postnatal doses) | Uncomplicated | No adverse events in the mother |
| Touraine et al (2004) | Haemophilia A | Fetal liver and thymic cells | ·· | 15 | ·· | 1 | Uncomplicated | No comment |
| Flake et al (1997) | Hurler syndrome | Fetal liver cells | Umbilical vein | 14 | ·· | 1 | Uncomplicated | No comment |
| Touraine et al (2004) | Niemann-Pick disease | Fetal liver cells | Intraperitoneally | 16, 18 | ·· | 2 | Uncomplicated | Mother in excellent condition |
| Le Blanc et al (2005), Götherström et al (2014) | Osteogenesis imperfecta | Fetal MSC | Umbilical vein | 31 | 5·0 × 106 per kg | 1 (+ postnatal doses) | Uncomplicated | No adverse events adverse events in the mother |
| Götherström et al (2014) | Osteogenesis imperfecta | Fetal MSC | Umbilical vein | 30 | 30·0 × 106 per kg | 1 (+ 1 postnatal dose) | Uncomplicated | No adverse events in the mother |
| JKY Chan, National University Hospital, Singapore | Osteogenesis imperfecta | Fetal MSC | Umbilical vein | 28 | 10·3 × 106 per kg | 1 (+ 1 postnatal dose) | Uncomplicated | No adverse events in the mother |
| Davis (1967) | Rhesus immunisation | Fetal MSC | Intraperitoneally | 11 | 1 mL of bone marrow | 1 | Uncomplicated | No comment (done via hysterotomy) |
| Linch et al (1986) | Rhesus immunisation | Fetal MSC | Umbilical vein | 17 | 1·8 × 108 cells per kg (the numbers of nucleated cells were 7–5 × 109 per kg and the number of colony-forming cells administered were 2–7 × 106 per kg) | 1 | Uncomplicated | No comment, done via fetoscopy with five additional blood transfusions in utero |
| Thilaganthan et al (1993) | Rhesus immunisation | Fetal bone marrow cells | Umbilical vein | 12 | 2·3 × 107 cells per kg | 1 | Uncomplicated | No comment |
| Wengler et al (1996) | Severe combined immunodeficiency | Fetal liver cells | Intraperitoneally | 21, 22 | 14 × 106 and 4 × 106 cells per kg | 2 | Uncomplicated | The injection procedure was well tolerated by the mother |
| Pirovano et al (2004) | Severe combined immunodeficiency | Fetal liver cells | Not provided | Between 21–26 | 20·0 × 106 cells per kg | ·· | Uncomplicated | No maternal engraftment at birth |
MSC= mesenchymal stem and progenitor cells.
Current developments in this field have focused on identifying the optimal modality of administration of in-utero stem-cell transplantation (IUT; such as intravenous injection into the vitelline or umbilical veins34) and on strategies for providing donor cells a competitive advantage over the fetal recipient’s endogenous stem cells. Providing donor cells with a competitive advantage has been identified as the major barrier to achieving therapeutic rates of engraftment following IUT. HSCs derived from adult bone marrow have been used extensively in experimental IUT, but engraftment rates have been subtherapeutic due to the fact that they are outcompeted for space in the fetal haematopoietic stem-cell niche by host equivalents. Attempts to modulate HSC proliferation kinetics before transplantation, or to ablate the fetal haematopoietic stem-cell niche before IUT, have had little success. Two strategies have been proposed that might have substantial translational potential. The first strategy involves the use of fetal donor cells for IUT such as amniotic fluid stem cells (AFSCs).35 AFSCs have been shown to have better engraftment potential compared with bone marrow-derived HSCs in murine models of IUT, and could be used in the autologous setting (with ex-vivo gene engineering),35 but substantial challenges remain to be addressed (eg, the requirement for expansion before IUT). The second strategy involves therapeutic cell engineering of bone marrow-derived HSCs using growth factor and small molecule drug-loaded nanoparticles. Release of these growth factors and small-molecule drugs from nanoparticles attached to the cell surface of donor HSCs allows persistent and targeted modulation of donor HSC proliferation kinetics in vivo (via a pseudo-autocrine mechanism), and might ameliorate the competitive advantage of the fetal host equivalents. A recent proof of principle study has shown that remarkable rates of long-term engraftment post experimental IUT can be achieved by decorating haematopoietic cells with GSK3 inhibitor-loaded nanoparticles, with the hope of allowing future single-step prenatal treatment of congenital haematological and other inherited disorders.36
There are currently two ongoing IUT clinical trials registered with ClinicalTrials.gov, one using HSCs to treat α-thalassaemia major (NCT02986698) and one using MSCs to treat severe osteoporosis imperfecta (NCT03706482).21 Without intervention, α-thalassaemia is fatal in utero, and the phase 1 clinical trial investigates the safety, feasibility, and efficacy of administering one dose of CD34+ enriched HSCs derived from maternal bone marrow to ten fetuses diagnosed with α-thalassaemia. The HSCs are administered into the umbilical vein between gestational weeks 18 and 25, at the same time as the intrauterine transfusion of red blood cells indicated for fetal anaemia treatment. The Boost Brittle Bones before Birth (BOOSTB4) trial uses first-trimester fetal liver-derived MSC IUT as a therapy for severe forms of osteoporosis imperfecta (type III and severe type IV). Previous case studies suggest that prenatal and postnatal transplantation of fetal MSCs is safe and efficient in this patient group,37 albeit with poor engraftment and a paracrine mechanism of action. In this phase 1–2 multicentre trial, both IUT and transplantation after birth are evaluated, and endpoints include safety, tolerability (for the mother, fetus, and infant), and efficacy.
In-utero gene therapy
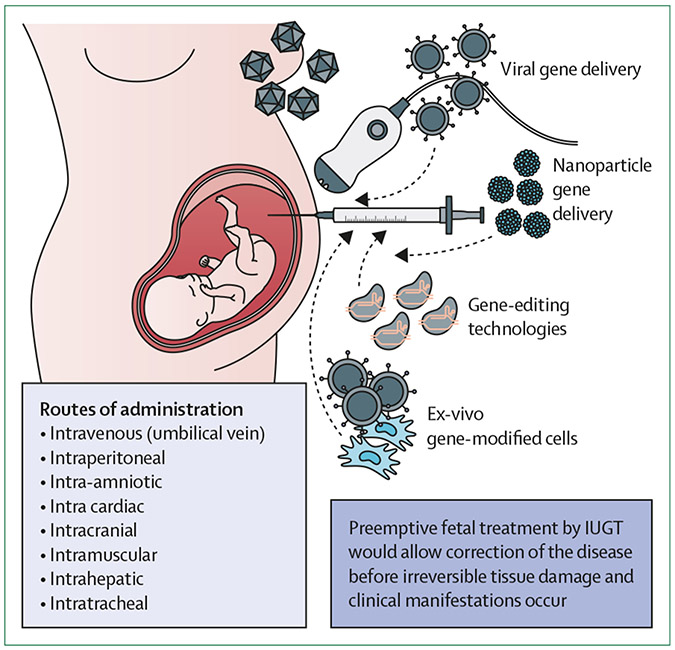
Multiple genetic disorders would be amenable to treatment by in-utero gene therapy (figure 2).30 Although results obtained with current postnatal gene therapy trials are encouraging,5,6 barriers to effective therapy include immune responses to the delivery vector or therapeutic protein, and initiation of treatment after disease onset in fetal life. Furthermore, the limited access to relevant numbers of fetal stem cells means that autologous ex-vivo therapy is probably of little use in those conditions where a fetal treatment is desired. Delivery of an in-vivo platform to the developing fetus poses additional requirements of safe fetal access, appropriate biodistribution, and targeted delivery. Specific to integrating platforms (predominantly lentiviruses), there are concerns regarding insertional mutagenesis.38 Furthermore, off-target effects of baseediting in vivo will require ongoing work assuring the specificity of CRISPR–Cas9 platforms,39 or by using alternative means of editing with reduced rates of mutagenesis.40
Figure 2: Prenatal gene therapy, gene editing, and gene-modified cell therapy.
In-utero gene therapy, gene editing, and gene-modified cell therapies are all viable options to provide a cure for monogenic diseases. Correcting the disease before birth has multiple potential advantages over postnatal treatment, including the ability to induce tolerance to foreign cells or proteins, prevent irreversible tissue damage, and deliver to multiple organs by using different routes of administration. IUGT=in-utero gene therapy.
Earlier prominent preclinical studies of in-utero gene therapy explored the use of in-utero gene addition. For example, in both murine and non-human primate models, prenatal delivery of human factor IX (hFIX) or human factor X (hFX) using direct administration of AAV vectors gave long-term curative plasma concentrations of hFIX or hFX, with no evidence of clinical toxicity.41 Similarly, a lentiviral approach has been used in a humanised mouse model of β-thalassaemia, in which the heterozygous mice, injected in utero with β-globin expressing lentivirus, showed phenotypic normalisation.38
Prenatal gene editing has been a focus of a number of recent studies. Bose and colleagues showed phenotypic rescue of a mouse model of Hurler syndrome by systemic prenatal delivery of a paired AAV2/9 vector bearing a CRISPR–Cas9 construct, which successfully base-edited the causative mutation.40 A similar AAV-based gene-editing approach was used for in-utero gene therapy of hereditary tyrosinaemia type I.42 The utility of non-viral (nanoparticle-based) platforms for delivery of in-utero gene therapy has also been shown. Ricciardi and colleagues reported delivery of a mutation-specific peptide-nucleic acid in a poly lactic-co-glycolic acid nanoparticle to a mouse model of β-thalassaemia, with phenotypic near normalisation.12
Preemptive fetal treatment by in-utero gene therapy might one day allow disease correction before irreversible tissue damage and clinical manifestations occur in multiple conditions.43 Examples include diseases such as neurometabolic disorders, cystic fibrosis, the haemoglobinopathies (all causing severe perinatal morbidity), and conditions in which early exposure to a missing protein would result in immunological tolerance (eg, haemophilia A). Gene delivery to treat genetic disorders before birth has been intensely discussed for decades. Reporter gene expression in multiple fetal tissues during gestation, at the rates required for therapeutic efficacy, was shown in the late 1990s. Additionally, the US National Institutes of Health Recombinant DNA Advisory Committee issued a statement on in-utero gene therapy, outlining the additional preclinical work that would be required for safe clinical translation. Ultimately, maternal and fetal safety are priorities;21 therefore, minimising the risk of trafficking to the mother and reducing off-target events have to be considered before allowing the use of in-utero gene therapy in clinical settings.
In-utero tissue-based therapy
There are a small number of fetal structural defects for which anatomical repair in the prenatal period is offered. The best example is myelomeningocele, for which there is level I evidence that operating before birth improves the outcome.44 The wide acceptance of this procedure boosted interest in the development of prenatal therapies for myelomenigocele but also highlighted the limitations of the procedure. Outcomes could be improved by better surgical technique (eg, to reduce tethering or prevent inclusion cysts) and earlier intervention. Indeed, the effects of fetal surgery seem to be time sensitive, with earlier spinal closure associated with superior walking ability.45 Very early in pregnancy, surgical repair is difficult with current instrumentation, so alternative strategies to cover the lesion have been proposed. These methods can be simple barriers formed by amniotic fluid or placental-derived MSCs, both of which are found to be safe and effective.46 Bioactive approaches to promote neuronal repair or an engineered functional tissue to cover large lesions have also been tested preclinically. Cell-free approaches such as alginate microparticles loaded with basic fibroblast growth factor induced tissue coverage in a rat model of myelomeningocele.47 Similarly, novel bioadhesive facilitates the delivery and attachment of alginate-polyacrylamide hydrogels to cover the spina bifida defect in a fetal rabbit model.48 Collagen scaffolds embedded with vascular endothelial growth factor and basic fibroblast growth factor could also be used to treat full-thickness fetal skin defects in sheep. Repairing and regenerating the neuronal placode have also been investigated by including stromal cells for modulation in immune-mediated local damage to the spinal cord, or neurons that would be integrated in vivo. Finally, fetal skin biodegradable collagen scaffolds can be used to treat full-thickness fetal skin defects,49 and to facilitate skin closure in fetuses undergoing prenatal repair in small and large animal models. Facilitating skin closure in fetuses undergoing prenatal repair might become useful to treat other prenatal defects such as gastroschisis, which could be treated before birth.
Although it is tempting to address congenital malformations with an anatomical repair, which is often feasible, it is not necessarily the optimal approach. One such example is congenital diaphragmatic hernia, in which anatomical prenatal repair was quickly abandoned for a procedure that focuses on the actual life-threatening factor of the condition—ie, pulmonary hypoplasia. Lung growth is stimulated by fetoscopic endoluminal tracheal occlusion, shown to be beneficial in fetuses with severe pulmonary hypoplasia, either right or left sided.50,51 Although fetoscopic endoluminal tracheal occlusion improves outcomes, results are still suboptimal, so adjuncts are being considered to further stimulate lung development.52 The administration of extracellular vesicles derived from amniotic fluid stem cells (AFSCs) has shown the ability to regenerate underdeveloped fetal lungs when delivered in preclinical animal models.53 In particular, when delivered intratracheally, administration of AFSC extracellular vesicles promoted branching morphogenesis and alveolarisation, rescued tissue homeostasis, and stimulated epithelial cell and fibroblast differentiation. These findings are in keeping with previous observations that intratracheal injection of AFSCs improved pulmonary development when combined with fetoscopic endoluminal tracheal occlusion in a rabbit model for congenital diaphragmatic hernia.54 Moreover, intravenous infusion of MSC-derived extracellular vesicles in a rodent congenital diaphragmatic hernia model attenuated pathological extracellular matrix and vasculature remodelling in the congenital diaphragmatic hernia pulmonary vasculature.55 Similarly, transamniotic delivery of stem cells can positively influence both lung maturation56 and lung vascular development in animals with induced congenital diaphragmatic hernias.57 Finally, stem-cell technology can also help with modelling diseases and finding new therapeutic options. In the context of congenital diaphragmatic hernias, in-vitro models have been described by using transgene-free human iPSCs generated from affected fetuses and infants.58
Immunological considerations
IUT was initially proposed as a method (1) to transplant allogeneic stem cells that can engraft; and (2) for which immune tolerance towards the donor cells in the primitive fetal immune system could be achieved without myeloablation, especially when transplanted during a window of opportunity in early gestation (prior to thymic maturation). However, a number of reports have since shown the presence of mature T-cells, functioning natural killer cells, and a fully developed antigenpresenting network at 12–14 weeks gestation,59,60 which can reject foreign cells.
Accumulating data suggest that donor-derived T-cells can support the achievement of clinically significant rates of donor cell engraftment in the fetus. Additionally, data from studies in animals suggest that low rates of donor cell engraftment can induce central fetal tolerance, which can be exploited in booster transplantation with minimal myeloablation postnatally. Furthermore, the use of fetal donor cells might offer some benefits compared with postnatal donor cells—ie, a fetal-to-fetal approach might result in higher engraftment rates and lower risk of graft-versus-host disease. The permissive immunological status at this gestational age would also probably mean a more permissive environment for the delivery of a transgene product or gene therapy vector with immunologenic potential.
The fetal immunological barrier is evident by the fact that clinical IUT using HSCs has been most successful in fetuses affected by immunodeficiency disorders who cannot reject the donor cells.30 However, by using MSCs that exhibit a low immunogenic profile, long-term, low-level engraftment has been achieved after IUT for osteoporosis imperfecta. The fetal barrier hampers the development of IUT, and new strategies must be developed alongside studies to further understand the fetal immune system,67 including manipulation of peripheral tolerance mechanisms that might extend the immunological window of opportunity.62
A final immunological consideration in prenatal therapy is that of the maternal immune system. Maternal alloimmunisation, triggered by the transplanted donor cells with subsequent transfer of alloantibodies to the fetus across the placenta, can affect the success of IUT.63 However, this finding originates from studies in mice, and it remains to be determined if the observation is also true in humans (but it has led to early translational attempts of matching donor cells to the mother). Pregnancy in itself poses an immunological challenge, because a genetically different fetus must be supported throughout gestation. This challenge is recognised to be a delicate balance of mutual tolerance to maintain a healthy pregnancy during infection, inflammation, or fetal stress, which will probably result in a common pathway of preterm labour, with evidence of fetal alloreactivity to the mother.64
Fetal cells for postnatal treatment
Advancement in early and specific prenatal diagnosis can help to tailor postnatal regenerative medicine treatments.65 Fetal cells have the advantage to be broadly multipotent—they can expand in large numbers and can easily be reprogrammed to pluripotent stem cells.66 This approach can be particularly valuable for conditions that do not necessarily need to be treated before birth, but that require surgical repair at birth. Cardiac malformations are a classic example, with engineered constructs prepared using fetal cells that are either harvested by direct biopsy of the fetus,67 or derived from pluripotent cells that have been reprogrammed from amniotic fluid and are undergoing functional cardiomyocyte differentiation.68 Using fetal cells derived from pluripotent cells is particularly exciting because of the potential to generate disease models that could help develop innovative treatments. Beside cardiac tissue, human AFSCs could be reprogrammed into vascular endothelial cells without transitioning through a pluripotent state,69 and they could be engineered in vitro into functional heart valves. Moreover, sheep AFSCs were seeded in biodegradable polyglycolic acid–poly-4-hydroxybutyrate composite matrices to engineer heart valves implanted orthotopically into the pulmonary position. The engineered valves showed in-vivo functionality with intact valvular integrity and an absence of thrombus formation.70 Similarly, AFSCs could be used to engineer full reprogramming (using myoblast determination protein 1 or by defined media) of skeletal muscles, which could help a functional repair of the diaphragm.71,72 AFSCs or human muscle progenitors can also be engineered in diaphragm-derived extracellular matrices and used to repair surgically-created diaphragmatic defects, and they have been shown to promote the generation of new blood vessels, boost long-term muscle regeneration, and recover host diaphragmatic function.73 Cells from different origins such as the lungs,74 kidneys,75 and liver have been isolated from amniotic fluid and could be used for therapy; however, susbtantial problems remain that limit clinical applications, namely associated with the low numbers of cells in vivo and the difficulties expanding these cells while maintaining stemness or efficiently obtaining functional differentiation. AFSCs and their vesicles have also shown immunomodulatory potential and have been proposed to rescue clinical features of necrotising enterocolitis,76 renal failure,77 hepatic failure,78 and lung fibrosis.79
Artificial placenta for ex-utero application of therapies to the fetus
Extreme prematurity remains the leading cause of infant morbidity and mortality worldwide. Advances in neonatal intensive care have increased survival of extremely premature infants, but interventions are associated with long-term morbidity.80 Despite these advances, the limit of viability of extreme prematurity sits at around 22 weeks, due to incomplete development of most major organ systems, especially the lungs. Thus, the concept of an extrauterine system to mimic the placental environment to support ongoing fetal growth and development would challenge our current boundaries of survival. This system could support extremely premature infants and provide an extrauterine environment for fetuses needing therapeutic intervention.
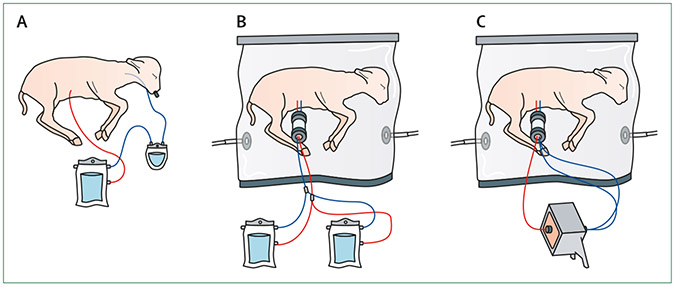
There are obvious obstacles to overcome in the design of an artificial placenta, such as the need for a circuit (either pumpless arteriovenous or pump-assisted venous–venous) with low resistance and a surface area oxygenator,81 while maintaining fluid-filled lungs. The provision of sterile fluid submersion and a supply of nutrition for fetal development are also important considerations.82 Some systems are based on a venovenous extracorporeal membrane oxygenation with a tracheal plug,83 whereas others use a pumpless arteriovenous circuit and a fluid environment that maintains fetal physiology. A few research groups have been working to develop an artificial placenta system to various degrees of success using prematurity animal models (figure 3).84,85 Despite this work, there are still substantial challenges to be overcome before clinical application can become a reality—for example, the need for swift transfer from the uterus to the artificial system, risks of haemorrhage and infection, and the optimal duration of support. For eventual clinical use of artificial placentas, they will need to support fetuses with early preterm premature rupture of the membranes or early-onset fetal growth restriction by delivering to an ex-utero setting. Furthermore, these systems should provide complete access to the fetal circulation independent of the mother for a sustained period of time, so that earlier repair of severe congenital defects or correction of genetic disorders via gene transfer or autologous stem-cell transplantation (with genetically corrected stem cells) can be achieved.
Figure 3: Current successful artificial placenta systems to support fetal growth in animal models.
The basic charateristics of an artificial placenta comprise of: an extracorporeal circuit; the maintanence of fetal circulation; fluid-filled lungs; and a womb-like environment for organ protection and development. (A) A venous–venous extracorporeal circuit with a pump.83 (B and C) These two systems use the umbilical vessels for access and have pumpless circuit. They also provide an extrauterine environment with a continuous exchange of amniotic fluid.84,85 (B) Uses two extracorporeal units.84 (C) Uses one extracorporeal unit.85
The concept of using artificial placentas to challenge the current threshold of extreme prematurity remains an issue full of ethical controversies. Nonetheless, we can foresee that the development and use of artificial placentas will be rapid, and additional regulation will be needed to ensure that any potential clinical applications are safe and ethical.
Looking into the near future
The current scientific advances and clinical applications of regenerative medicine make it an exciting, rapidly evolving field with great potential for the treatment of congenital disease before birth. Increased knowledge on prognosis and long-term outcomes, coupled with the rapid evolution of diagnostic tools and therapeutic instrumentations, have made the safe and effective prenatal delivery of regenerative therapies possible. Administration of treatment before the development of permanent tissue injury or damage—as well as fetal size, immunological immaturity, and healing potential—are some of the clear advantages of intervention before the neonatal period. Although major technical and ethical limitations remain (including the risks of treatment complications in both the mother and the fetus), the evolution of platforms such as the artificial placenta could facilitate the in-utero application of regenerative therapies. Many breakthroughs are still to be made within the preclinical arena before the full potential of regenerative medicine can be realised for prenatal therapy, but some of these therapeutic platforms will be deployed to the fetus in the very near future.
Key messages.
Recent advances in regenerative medicine are ideally suited to treat congenital diseases that currently do not have effective therapies
Understanding and subsequent successes in disease modelling and novel gene, cell, cell-free, and tissue-based therapies of rare paediatric diseases have led to first-in-human breakthroughs, laying the foundation for clinical trials
Advances in paediatric regenerative medicine have inspired applications in some adult conditions
Prenatal intervention provides multiple advantages but also presents biomedical, technological, and ethical challenges
Search strategy and selection criteria.
We searched Web of Science and PubMed for reports in English from Oct 1, 2011, to Sept 1, 2021, using the search terms “congenital diseases”, “paediatric diseases”, “regenerative medicine”, “stem cell therapy”, “gene therapy’, “gene editing”, “cell-free therapy”, “in utero therapy”, “prenatal diagnosis”, “fetal therapy”, “tissue engineering”, and “artificial placenta”. Some older references were also included owing to their importance. Because of restrictions in the number of references allowed, review articles were chosen where appropriate to provide readers with more details and further references to some worthy, but older, original articles.
Acknowledgments
PKHT’s work on Hirschsprung’s disease and biliary atresia is supported by the Research Grants Council, Hong Kong (T12C-747/14, HKU171195/14, HKU17109918, C7139-20GF, T12-712/21-R), the Health and Medical Research Fund, Hong Kong (03143476, PR-HKU-1, 08192376) and the Innovation and Technology Fund (UIM 299, 300). PDC is supported by the UK National Institute for Health Research (NIHR-RP-2014-04-046), Great Ormond Street Hospital Children’s Charity, and the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust. JKYC is supported by Singapore’s Ministry of Health’s National Medical Research Council (NMRC/CSA-SI-008-2016, CIRG-1484-2018). ALD is supported by the NIHR Biomedical Research Centre at University College London Hospital. CG and ALD are supported by funding from the EU Horizon 2020 research and innovation programme under grant agreement no. 681045.
Footnotes
Declaration of interests
PKHT reports fee from BlueRock Therapeutics and Xellera Therapeutics.
Contributor Information
Paolo de Coppi, Stem Cell and Regenerative Medicine Section, Department of Developmental Biology and Cancer Research and Teaching; Department of Specialist Neonatal and Paediatric Surgery.
Stavros Loukogeorgakis, Stem Cell and Regenerative Medicine Section, Department of Developmental Biology and Cancer Research and Teaching; Department of Specialist Neonatal and Paediatric Surgery.
Cecilia Götherström, Great Ormond Street Institute of Child Health, University College London, London, UK; Department of Clinical Science, Intervention and Technology, Karolinska Institute, Stockholm, Sweden.
Anna L David, Elizabeth Garrett Anderson Institute for Womens Health, University College London, London, UK.
Graça Almeida-Porada, Wake Forest Institute for Regenerative Medicine, Fetal Research and Therapy Program, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem NC, USA.
Jerry K Y Chan, Academic Clinical Program in Obstetrics and Gynaecology, Duke-NUS Medical School, Singapore; Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore.
Jan Deprest, Clinical Department of Obstetrics and Gynaecology, UZ Leuven, Leuven, Belgium.
Kenneth Kak Yuen Wong, Division of Paediatric Surgery, Department of Surgery, Queen Mary Hospital, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pok Fu Lam, Hong Kong, Special Administrative Region, China.
Paul Kwong Hang Tam, Division of Paediatric Surgery, Department of Surgery, Queen Mary Hospital, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pok Fu Lam, Hong Kong, Special Administrative Region, China; Faculty of Medicine, Macau University of Science and Technology, Macau Special Administrative Region, China.
References
- 1.Staal FJT, Aiuti A, Cavazzana M. Autologous stem-cell-based gene therapy for inherited disorders: state of the art and perspectives. Front Pediatr 2019; 7: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkers G, van Mourik P, Vonk AM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep 2019; 26: 1701–08. [DOI] [PubMed] [Google Scholar]
- 3.Manfroi S, Calisesi C, Fagiani P, et al. Prenatal non-invasive foetal RHD genotyping: diagnostic accuracy of a test as a guide for appropriate administration of antenatal anti-D immunoprophylaxis. Blood Transfus 2018; 16: 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Li J, Saucier JB, et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. Nat Med 2019; 25: 439–47. [DOI] [PubMed] [Google Scholar]
- 5.Kanter J, Walters MC, Krishnamurti L, et al. Biologic and clinical efficacy of lentiglobin for sickle cell disease. N Engl J Med 2022; 386: 617–28. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Thompson AA, Kwiatkowski JL, et al. Betibeglogene autotemcel gene therapy for non-β0/β0 genotype β-thalassemia. N Engl J Med 2022; 386: 415–27. [DOI] [PubMed] [Google Scholar]
- 7.Nitkin CR, Bonfield TL. Concise Review: Mesenchymal stem cell therapy for pediatric disease: perspectives on success and potential improvements. Stem Cells Transl Med 2017; 6: 539–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh RE, Berild JD, Sterrantino AF, Toledano MB, Hansell AL. Birth weight trends in England and Wales (1986-2012): babies are getting heavier. Arch Dis Child Fetal Neonatal Ed 2018; 103: F264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendriks D, Artegiani B, Hu H, Chuva de Sousa Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat Protoc 2021; 16: 182–217. [DOI] [PubMed] [Google Scholar]
- 10.Palanki R, Peranteau WH, Mitchell MJ. Delivery technologies for in utero gene therapy. Adv Drug Deliv Rev 2021; 169: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doudna JA. The promise and challenge of therapeutic genome editing. Nature 2020; 578: 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricciardi AS, Bahal R, Farrelly JS, et al. In utero nanoparticle delivery for site-specific genome editing. Nat Commun 2018; 9: 2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witman N, Zhou C, Grote Beverborg N, Sahara M, Chien KR. Cardiac progenitors and paracrine mediators in cardiogenesis and heart regeneration. Semin Cell Dev Biol 2020; 100: 29–51. [DOI] [PubMed] [Google Scholar]
- 14.Hirai K, Ousaka D, Fukushima Y, et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med 2020; 12: eabb3336. [DOI] [PubMed] [Google Scholar]
- 15.Mathews SJ, Shakir DI, Mosse CA, et al. Ultrasonic needle tracking with dynamic electronic focusing. Ultrasound Med Biol 2022; 48: 520–29. [DOI] [PubMed] [Google Scholar]
- 16.Bano S, Vasconcelos F, Tella-Amo M, et al. Deep learning-based fetoscopic mosaicking for field-of-view expansion. Int J CARS 2020; 15: 1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiro JL, Fontecha CG, Ruano R, et al. Single-Access Fetal Endoscopy (SAFE) for myelomeningocele in sheep model I: amniotic carbon dioxide gas approach. Surg Endosc 2013; 27: 3835–40. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad MA, Ourak M, Gruijthuijsen C, Deprest J, Vercauteren T, Vander Poorten E. Deep learning-based monocular placental pose estimation: towards collaborative robotics in fetoscopy. Int J CARS 2020; 15: 1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheppard M, Spencer RN, Ashcroft R, David AL, EVERREST Consortium. Ethics and social acceptability of a proposed clinical trial using maternal gene therapy to treat severe early-onset fetal growth restriction. Ultrasound Obstet Gynecol 2016; 47: 484–91. [DOI] [PubMed] [Google Scholar]
- 20.Hill M, Lewis C, Riddington M, et al. Stakeholder views and attitudes towards prenatal and postnatal transplantation of fetal mesenchymal stem cells to treat osteogenesis imperfecta. Eur J Hum Genet 2019; 27: 1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagar R, Almeida-Porada G, Blakemore K, et al. Fetal and maternal safety considerations for in utero therapy clinical trials: iFeTiS consensus statement. Mol Ther 2020; 28: 2316–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwiers C, Lindenburg ITM, Klumper FJ, de Haas M, Oepkes D, Van Kamp IL. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound Obstet Gynecol 2017; 50: 180–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattar CNZ, Tan YW, Johana N, et al. Fetoscopic versus ultrasound-guided intravascular delivery of maternal bone marrow cells in fetal macaques: a technical model for intrauterine haemopoietic cell transplantation. Fetal Diagn Ther 2019; 46: 175–86. [DOI] [PubMed] [Google Scholar]
- 24.Spencer RN, Hecher K, Norman G, et al. Development of standard definitions and grading for maternal and fetal adverse event terminology. Prenat Diagn 2022; 42: 15–26. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence. Preterm labour and birth. 2022. https://www.nice.org.uk/guidance/ng25 (accessed July 8, 2022). [PubMed] [Google Scholar]
- 26.Lo YM. Non-invasive prenatal testing using massively parallel sequencing of maternal plasma DNA: from molecular karyotyping to fetal whole-genome sequencing. Reprod Biomed Online 2013; 27: 593–98. [DOI] [PubMed] [Google Scholar]
- 27.Xiong L, Barrett AN, Hua R, et al. Non-invasive prenatal diagnostic testing for β-thalassaemia using cell-free fetal DNA and next generation sequencing. Prenat Diagn 2015; 35: 258–65. [DOI] [PubMed] [Google Scholar]
- 28.Xiang Y, Zhang J, Li Q, et al. DNA methylome profiling of maternal peripheral blood and placentas reveal potential fetal DNA markers for non-invasive prenatal testing. Mol Hum Reprod 2014; 20: 875–84. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead CL, Walker SP, Tong S. Measuring circulating placental RNAs to non-invasively assess the placental transcriptome and to predict pregnancy complications. Prenat Diagn 2016; 36: 997–1008. [DOI] [PubMed] [Google Scholar]
- 30.Almeida-Porada G, Atala A, Porada CD. In utero stem cell transplantation and gene therapy: rationale, history, and recent advances toward clinical application. Mol Ther Methods Clin Dev 2016; 5: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derderian SC, Jeanty C, Walters MC, Vichinsky E, MacKenzie TC. In utero hematopoietic cell transplantation for hemoglobinopathies. Front Pharmacol 2015; 5: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekblad-Nordberg Å, Walther-Jallow L, Westgren M, Götherström C. Prenatal stem cell therapy for inherited diseases: past, present, and future treatment strategies. Stem Cells Transl Med 2020; 9: 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai-MacArthur S, Lombardi G, Shangaris P. The theoretical basis of in utero hematopoietic stem cell transplantation and its use in the treatment of blood disorders. Stem Cells Dev 2021; 30: 49–58. [DOI] [PubMed] [Google Scholar]
- 34.Boelig MM, Kim AG, Stratigis JD, et al. The intravenous route of injection optimizes engraftment and survival in the murine model of in utero hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22: 991–99. [DOI] [PubMed] [Google Scholar]
- 35.Loukogeorgakis SP, Shangaris P, Bertin E, et al. In utero transplantation of expanded autologous amniotic fluid stem cells results in long-term hematopoietic engraftment. Stem Cells 2019; 37: 1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loukogeorgakis SP, Fachin CG, Dias AIBS, et al. Donor cell engineering with GSK3 inhibitor-loaded nanoparticles enhances engraftment after in utero transplantation. Blood 2019; 134: 1983–95. [DOI] [PubMed] [Google Scholar]
- 37.Götherström C, Westgren M, Shaw SW, et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med 2014; 3: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shangaris P, Loukogeorgakis SP, Subramaniam S, et al. In utero gene therapy (IUGT) using GLOBE lentiviral vector phenotypically corrects the heterozygous humanised mouse model and its progress can be monitored using MRI techniques. Sci Rep 2019; 9: 11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JS, Dagdas YS, Kleinstiver BP, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 2017; 550: 407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bose SK, White BM, Kashyap MV, et al. In utero adenine base editing corrects multi-organ pathology in a lethal lysosomal storage disease. Nat Commun 2021; 12: 4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan JKY, Gil-Farina I, Johana N, et al. Therapeutic expression of human clotting factors IX and X following adeno-associated viral vector-mediated intrauterine gene transfer in early-gestation fetal macaques. FASEB J 2019; 33: 3954–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossidis AC, Stratigis JD, Chadwick AC, et al. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat Med 2018; 24: 1513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peranteau WH, Flake AW. The future of in utero gene therapy. Mol Diagn Ther 2020; 24: 135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peralta CFA, Botelho RD, Romano ER, et al. Fetal open spinal dysraphism repair through a mini-hysterotomy: influence of gestational age at surgery on the perinatal outcomes and postnatal shunt rates. Prenat Diagn 2020; 40: 689–97. [DOI] [PubMed] [Google Scholar]
- 46.Farrelly JS, Bianchi AH, Ricciardi AS, et al. Alginate microparticles loaded with basic fibroblast growth factor induce tissue coverage in a rat model of myelomeningocele. J Pediatr Surg 2019; 54: 80–85. [DOI] [PubMed] [Google Scholar]
- 47.Lazow SP, Labuz DF, Freedman BR, et al. A novel two-component, expandable bioadhesive for exposed defect coverage: applicability to prenatal procedures. J Pediatr Surg 2021; 56: 165–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosper NA, Eggink AJ, Roelofs LA, et al. Intra-uterine tissue engineering of full-thickness skin defects in a fetal sheep model. Biomaterials 2010; 31: 3910–19. [DOI] [PubMed] [Google Scholar]
- 49.Mazzone L, Moehrlen U, Ochsenbein-Kölble N, et al. Bioengineering and in utero transplantation of fetal skin in the sheep model: a crucial step towards clinical application in human fetal spina bifida repair. J Tissue Eng Regen Med 2020; 14: 58–65. [DOI] [PubMed] [Google Scholar]
- 50.Deprest JA, Benachi A, Gratacos E, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med 2021; 385: 119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deprest JA, Nicolaides KH, Benachi A, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med 2021; 385: 107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo FM, Cunha M, Jimenez J, et al. Complementary effect of maternal sildenafil and fetal tracheal occlusion improves lung development in the rabbit model of congenital diaphragmatic hernia. Ann Surg 2020; 275: e586–95. [DOI] [PubMed] [Google Scholar]
- 53.Antounians L, Catania VD, Montalva L, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med 2021; 13: eaax5941. [DOI] [PubMed] [Google Scholar]
- 54.DeKoninck P, Toelen J, Roubliova X, et al. The use of human amniotic fluid stem cells as an adjunct to promote pulmonary development in a rabbit model for congenital diaphragmatic hernia. Prenat Diagn 2015; 35: 833–40. [DOI] [PubMed] [Google Scholar]
- 55.Monroe MN, Zhaorigetu S, Gupta VS, et al. Extracellular vesicles influence the pulmonary arterial extracellular matrix in congenital diaphragmatic hernia. Pediatr Pulmonol 2020; 55: 2402–11. [DOI] [PubMed] [Google Scholar]
- 56.Takayama S, Sakai K, Fumino S, et al. An intra-amniotic injection of mesenchymal stem cells promotes lung maturity in a rat congenital diaphragmatic hernia model. Pediatr Surg Int 2019; 35: 1353–61. [DOI] [PubMed] [Google Scholar]
- 57.Chalphin AV, Lazow SP, Labuz DF, et al. Transamniotic stem cell therapy for experimental congenital diaphragmatic hernia: structural, transcriptional, and cell kinetics analyses in the nitrofen model. Fetal Diagn Ther 2021; 48: 381–91. [DOI] [PubMed] [Google Scholar]
- 58.Kunisaki SM, Jiang G, Biancotti JC, et al. Human induced pluripotent stem cell-derived lung organoids in an ex vivo model of the congenital diaphragmatic hernia fetal lung. Stem Cells Transl Med 2021; 10: 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alhajjat AM, Strong BS, Lee AE, et al. Prenatal allospecific NK cell tolerance hinges on instructive allorecognition through the activating receptor during development. J Immunol 2015; 195: 1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 2006; 6: 127–35. [DOI] [PubMed] [Google Scholar]
- 61.Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood 2006; 108: 4268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley JS, McClain LE, Stratigis JD, et al. Regulatory T cells promote alloengraftment in a model of late-gestation in utero hematopoietic cell transplantation. Blood Adv 2020; 4: 1102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merianos DJ, Tiblad E, Santore MT, et al. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest 2009; 119: 2590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halkias J, Rackaityte E, Hillman SL, et al. CD161 contributes to prenatal immune suppression of IFNγ-producing PLZF+ T cells. J Clin Invest 2019; 129: 3562–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davidson JR, Uus A, Matthew J, et al. Fetal body MRI and its application to fetal and neonatal treatment: an illustrative review. Lancet Child Adolesc Health 2021; 5: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins KE, Moschidou D, Faccenda D, et al. Human amniocytes are receptive to chemically induced reprogramming to pluripotency. Mol Ther 2017; 25: 427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuchs JR, Nasseri BA, Vacanti JP, Fauza DO. Postnatal myocardial augmentation with skeletal myoblast-based fetal tissue engineering. Surgery 2006; 140: 100–07. [DOI] [PubMed] [Google Scholar]
- 68.Jiang G, Herron TJ, Di Bernardo J, Walker KA, O’Shea KS, Kunisaki SM. Human cardiomyocytes prior to birth by integration-free reprogramming of amniotic fluid cells. Stem Cells Transl Med 2016; 5: 1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell 2012; 151: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weber B, Emmert MY, Behr L, et al. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials 2012; 33: 4031–43. [DOI] [PubMed] [Google Scholar]
- 71.Kim JA, Shon YH, Lim JO, Yoo JJ, Shin H-I, Park EK. MYOD mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury. Stem Cell Res Ther 2013; 4: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Coppi P, Bartsch G Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25: 100–06. [DOI] [PubMed] [Google Scholar]
- 73.Trevisan C, Maghin E, Dedja A, et al. Allogenic tissue-specific decellularized scaffolds promote long-term muscle innervation and functional recovery in a surgical diaphragmatic hernia model. Acta Biomater 2019; 89: 115–25. [DOI] [PubMed] [Google Scholar]
- 74.Lesage F, Zia S, Jiménez J, Deprest J, Toelen J. The amniotic fluid as a source of mesenchymal stem cells with lung-specific characteristics. Prenat Diagn 2017; 37: 1093–99. [DOI] [PubMed] [Google Scholar]
- 75.Da Sacco S, Perin L, Sedrakyan S. Amniotic fluid cells: current progress and emerging challenges in renal regeneration. Pediatr Nephrol 2018; 33: 935–45. [DOI] [PubMed] [Google Scholar]
- 76.Zani A, Cananzi M, Fascetti-Leon F, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 2014; 63: 300–09. [DOI] [PubMed] [Google Scholar]
- 77.Baulier E, Favreau F, Le Corf A, et al. Amniotic fluid-derived mesenchymal stem cells prevent fibrosis and preserve renal function in a preclinical porcine model of kidney transplantation. Stem Cells Transl Med 2014; 3: 809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng YB, Zhang XH, Huang ZL, et al. Amniotic-fluid-derived mesenchymal stem cells overexpressing interleukin-1 receptor antagonist improve fulminant hepatic failure. PLoS One 2012; 7: e41392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia O, Carraro G, Turcatel G, et al. Amniotic fluid stem cells inhibit the progression of bleomycin-induced pulmonary fibrosis via CCL2 modulation in bronchoalveolar lavage. PLoS One 2013; 8: e71679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson JG, Baer RJ, Partridge JC, et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics 2016; 138: e20154434. [DOI] [PubMed] [Google Scholar]
- 81.Schoberer M, Arens J, Erben A, et al. Miniaturization: the clue to clinical application of the artificial placenta. Artif Organs 2014; 38: 208–14. [DOI] [PubMed] [Google Scholar]
- 82.Morton SU, Brodsky D. Fetal physiology and the transition to extrauterine life. Clin Perinatol 2016; 43: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gray BW, El-Sabbagh A, Rojas-Pena A, et al. Development of an artificial placenta IV: 24 hour venovenous extracorporeal life support in premature lambs. ASAIO J 2012; 58: 148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Usuda H, Watanabe S, Saito M, et al. Successful use of an artificial placenta-based life support system to treat extremely preterm ovine fetuses compromised by intrauterine inflammation. Am J Obstet Gynecol 2020; 223: e1–20. [DOI] [PubMed] [Google Scholar]
- 85.Partridge EA, Davey MG, Hornick MA, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun 2017; 8: 15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tam PKH, Wong KKY, Atala A, et al. Postnatal approaches to regenerative medicine. Lancet Child Adolesc Health 2022; [Prod: please fill in—paper 21tlchild1350] [DOI] [PubMed] [Google Scholar]