Abstract
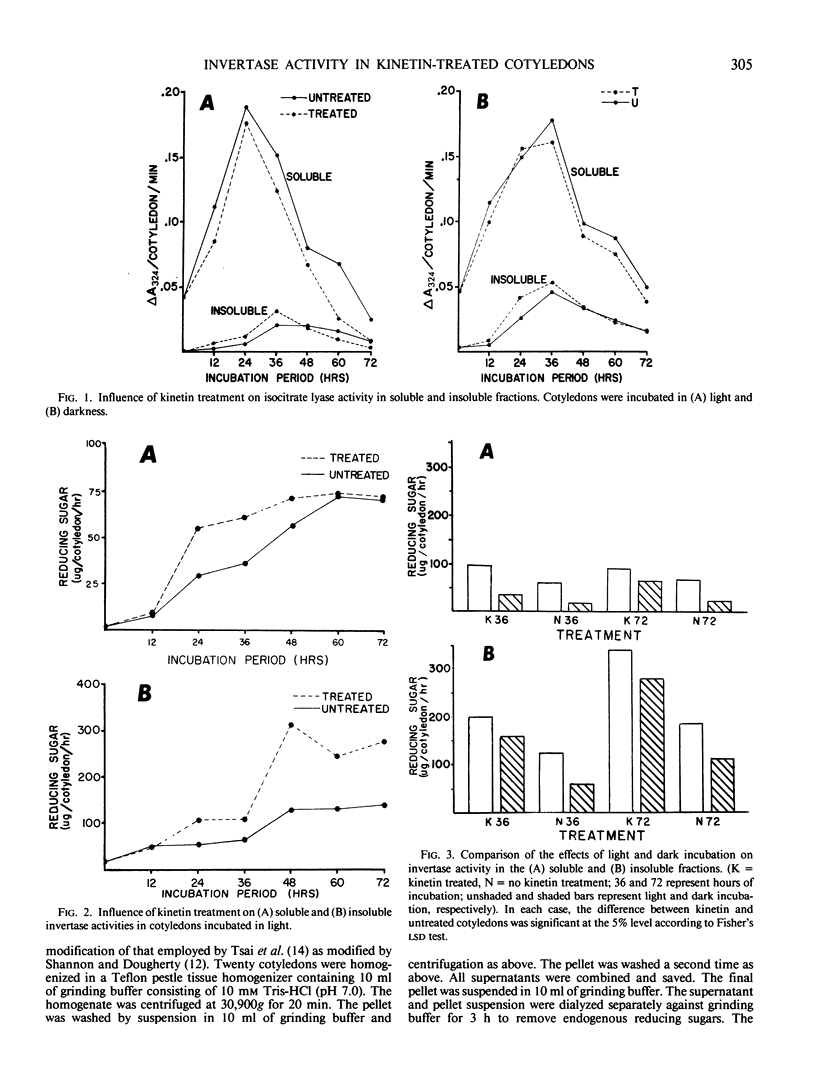
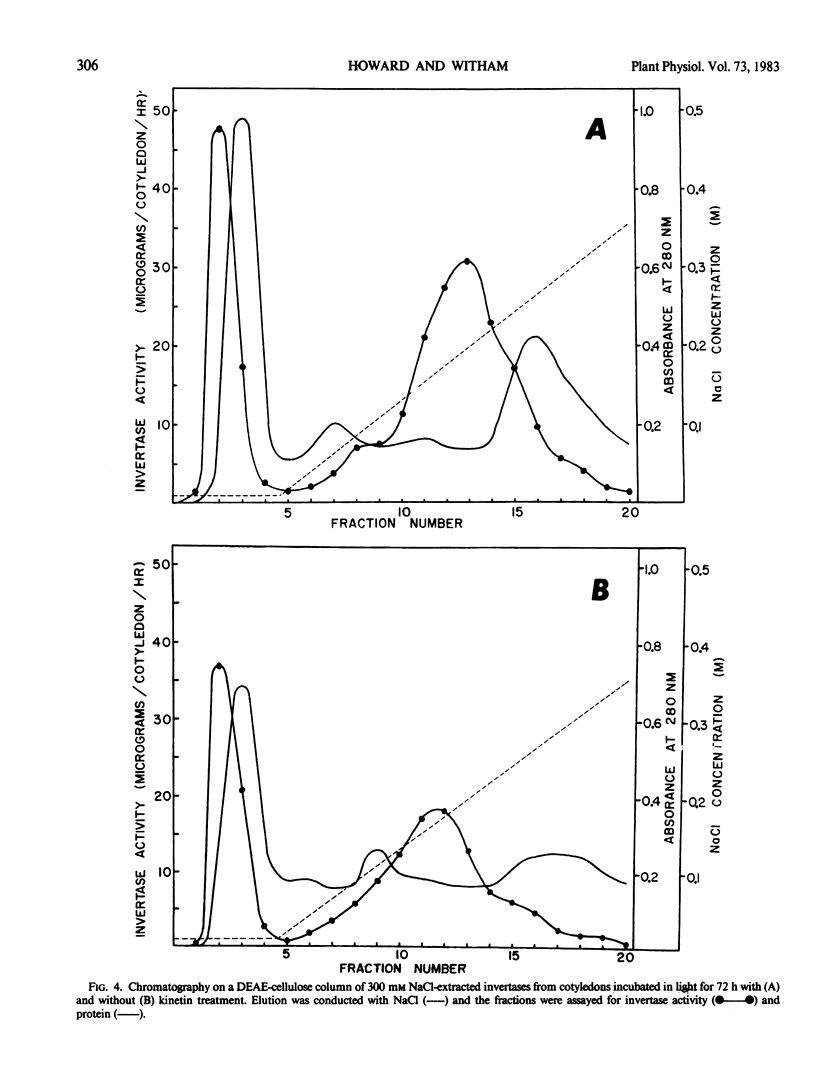
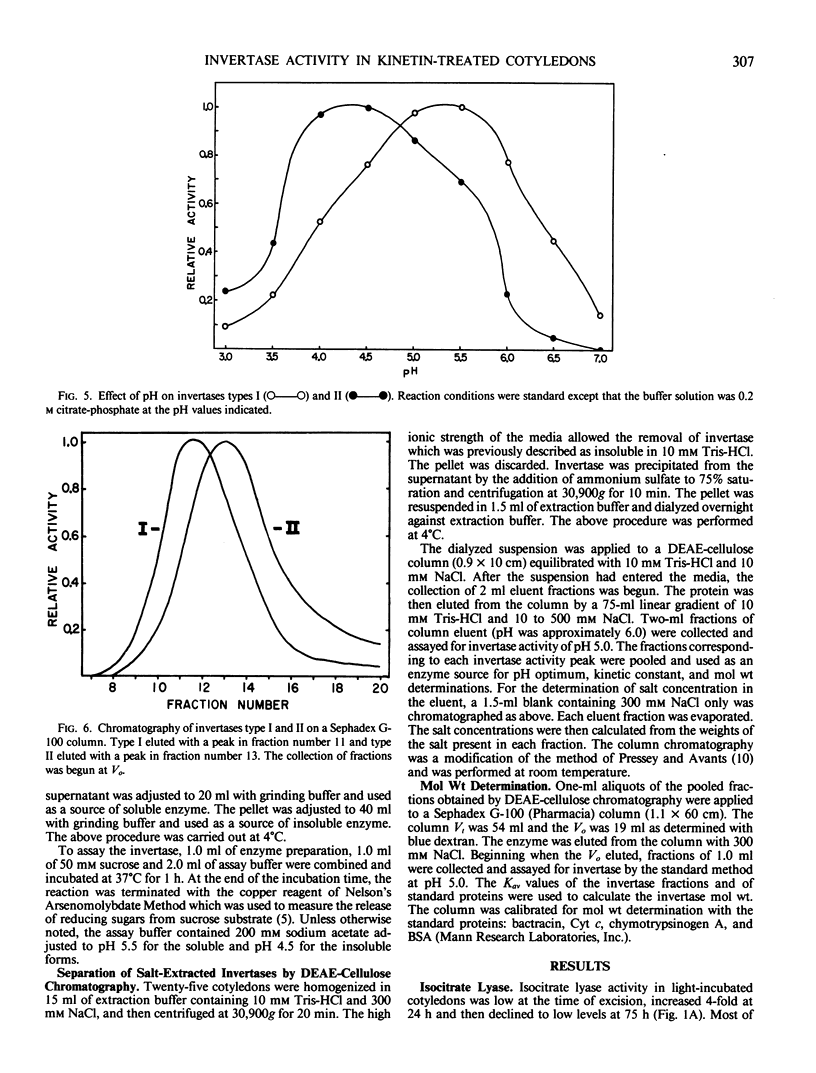
Cytokinin treatment is known to promote expansion of light-grown excised radish (Raphanus sativus L. cv Crimson Giant) cotyledons. This expansion, at least in part, seems to be related to an increased accumulation of osmotically active reducing sugars. Kinetin treatment did not cause increased levels of isocitrate lyase activity over the controls, but stimulated increased levels of two invertase forms, designated types I and II. Type I was soluble and type II was insoluble after homogenization in 10 millimolar tris(hydroxymethyl)aminomethane-HCl (pH 7.0). Both types were soluble after homogenization in 300 millimolar NaCl. At low salt concentration, type II was retained on a diethylamioethyl-cellulose column and type I was not. Type II was then eluted from the column at high salt concentration. Types I and II exhibited pH optima of 5.3 and 4.3, Michaelis constants of 4.96 and 1.23 millimolar sucrose, and molecular weights of 65,000 and 57,000 daltons, respectively. The kinetin promotion of reducing sugar accumulation may be related to increased levels of the two invertase forms, but is probably not a result of direct cytokinin-stimulated glyoxysomal activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Huff A. K., Ross C. W. Promotion of radish cotyledon enlargement and reducing sugar content by zeatin and red light. Plant Physiol. 1975 Sep;56(3):429–433. doi: 10.1104/pp.56.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner D., Ashton F. M. Hormonal control of isocitrate lyase synthesis. Biochim Biophys Acta. 1967 Nov 28;148(2):481–485. doi: 10.1016/0304-4165(67)90145-6. [DOI] [PubMed] [Google Scholar]
- Pressey R., Avants J. K. Invertases in Oat Seedlings: SEPARATION, PROPERTIES, AND CHANGES IN ACTIVITIES IN SEEDLING SEGMENTS. Plant Physiol. 1980 Jan;65(1):136–140. doi: 10.1104/pp.65.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijven A. H., Parkash V. Action of kinetin on cotyledons of fenugreek. Plant Physiol. 1971 Jan;47(1):59–64. doi: 10.1104/pp.47.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: II. Invertase Activity of the Pedicel and Placento-Chalazal Tissues. Plant Physiol. 1972 Feb;49(2):203–206. doi: 10.1104/pp.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. Y., Salamini F., Nelson O. E. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970 Aug;46(2):299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


