Abstract
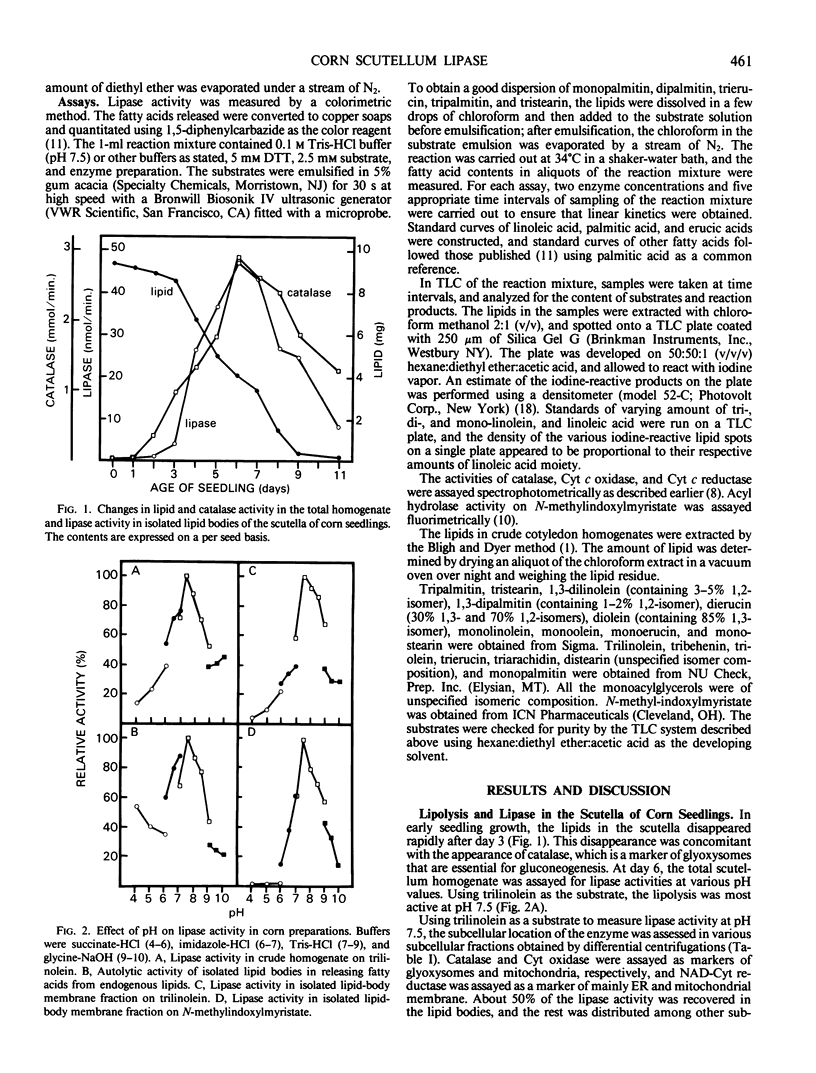
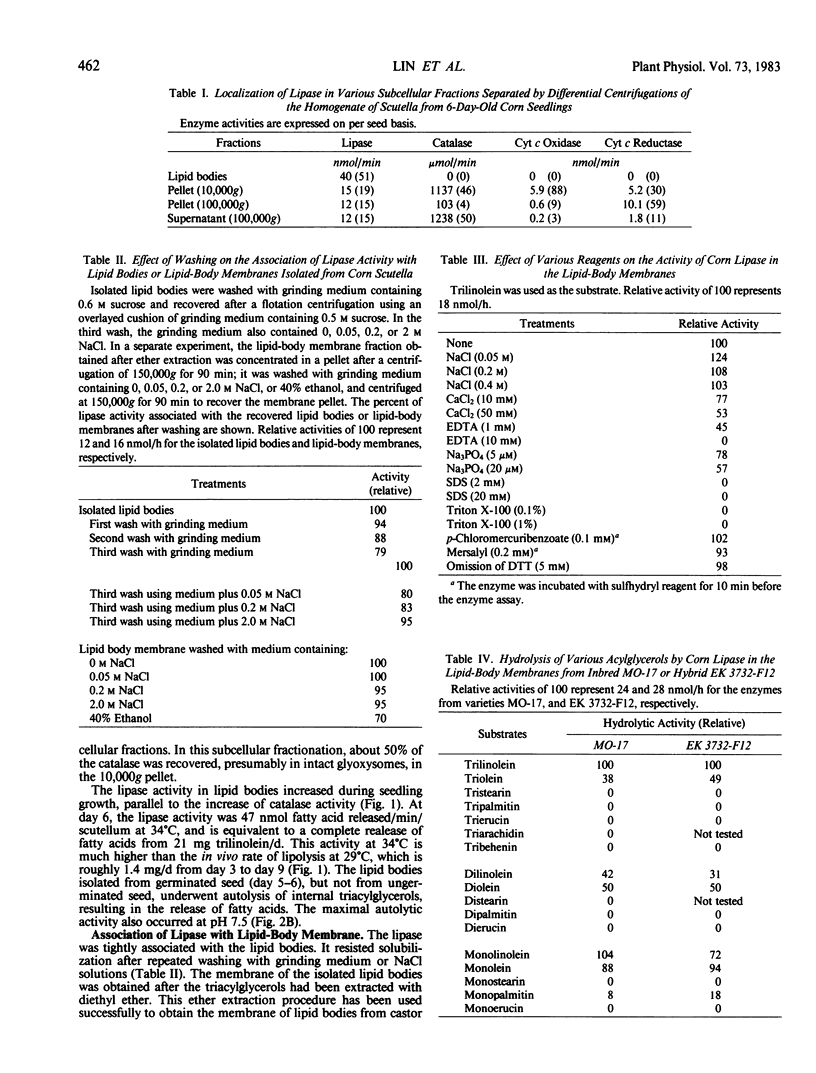
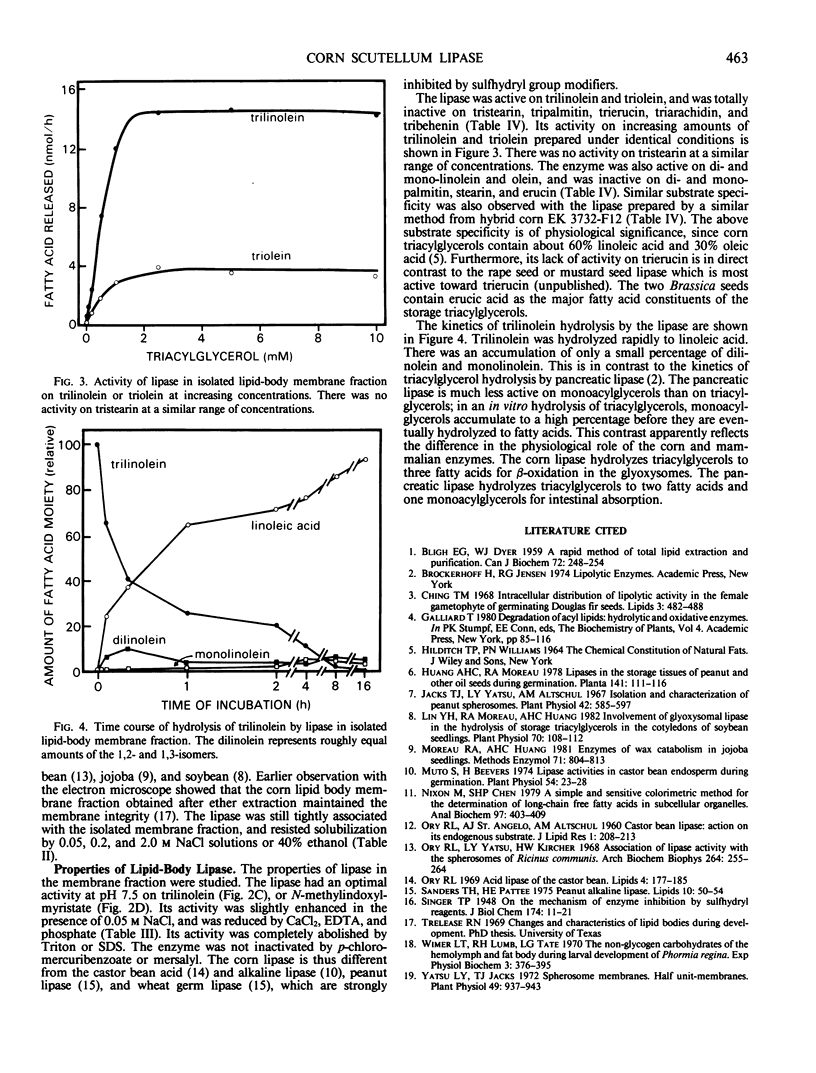
In the scutella of corn (Zea mays), lipase activity is absent in ungerminated seeds and increases during seedling growth. At the peak stage of lipolysis, about 50% of the lipase activity is recovered in the lipid body fraction after flotation centrifugation. The lipase is tightly bound to the lipid bodies, and resists solubilization by repeated washing with buffers or NaCl solutions. Isolated lipid bodies undergo autolysis of internal triacylglycerols, resulting in the release of fatty acids. After the triacylglycerols in isolated lipid bodies have been extracted with diethyl ether, the lipase is recovered in the membrane fraction. The lipase has an optimal activity at pH 7.5 in the autolysis of lipid bodies, or on trilinolein or N-methylindoxylmyristate. Of the various acylglycerols examined, the enzyme is active only on acylglycerols of linoleic and oleic acids which are the major fatty acid constituents of corn oil. The activity is not greatly affected by NaCl, CaCl2, or pretreatment of the enzyme with p-chloromercuribenzoate or mersalyl, and detergents abolish the activity. The enzyme hydrolyzes trilinolein completely to fatty acids; during the course of reaction, there is little accumulation of di- or mono-linolein.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ching T. M. Intracellular distribution of lipolytic activity in the female gametophyte of germinating Douglas fir seeds. Lipids. 1968 Nov;3(6):482–488. doi: 10.1007/BF02530890. [DOI] [PubMed] [Google Scholar]
- Jacks T. J., Yatsu L. Y., Altschul A. M. Isolation and characterization of peanut spherosomes. Plant Physiol. 1967 Apr;42(4):585–597. doi: 10.1104/pp.42.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H., Moreau R. A., Huang A. H. Involvement of glyoxysomal lipase in the hydrolysis of storage triacylglycerols in the cotyledons of soybean seedlings. Plant Physiol. 1982 Jul;70(1):108–112. doi: 10.1104/pp.70.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon M., Chan S. H. A simple and sensitive colorimetric method for the determination of long-chain free fatty acids in subcellular organelles. Anal Biochem. 1979 Sep 1;97(2):403–409. doi: 10.1016/0003-2697(79)90093-9. [DOI] [PubMed] [Google Scholar]
- ORY R. L., ST ANGELO A. J., ALTSCHUL A. M. Castor bean lipase: action on its endogenous substrate. J Lipid Res. 1960 Apr;1:208–213. [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Sanders T. H., Pattee H. E. Peanut alkaline lipase. Lipids. 1975 Jan;10(1):50–54. doi: 10.1007/BF02532194. [DOI] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]


