Abstract
Routine usage of antibiotics for animal health is a key driver of antimicrobial resistance (AMR) in food-producing animals. Taxation is a possible approach to incentivise appropriate antibiotic usage in food-producing animals. Taxation can be applied flatly across all antibiotic classes, targeted to single antibiotic classes, or scaled based on resistance in each class, so called “differential” taxation. However, quantifying the potential impact of taxation is challenging, due to the nonlinear and unintuitive response of AMR dynamics to interventions and changes in antibiotic usage caused by alterations in price. We combine epidemiological models with price elasticities of demand for veterinary antibiotics, to compare the potential benefits of taxation schemes with currently implemented bans on antibiotic usage.
Taxation strategies had effects comparable to bans on antibiotic usage in food-producing animals to reduce average resistance prevalence and prevent increases in overall infection. Taxation could also maximise the average number of antibiotics with a resistance prevalence of under 25% and potentially generate annual global revenues of ∼1 billion US$ under a 50% taxation to current prices of food-producing animal antibiotics. Differential taxation was also able to maintain a high availability of antibiotics over time compared to single and flat taxation strategies, while also having the lowest rates of intervention failure and highest potential revenue across all taxation strategies. These findings suggest that taxation should be further explored as a tool to combat the ongoing AMR crisis.
Keywords: Antimicrobial resistance, Taxation, Mathematical model, Intervention, One health, Food-producing animals
Highlights
-
•
Taxation can be as effective as bans on antibiotic usage in food-producing animals to reduce antimicrobial resistance.
-
•
Taxation can generate ∼1 billion USD under a 50% increase in antibiotic price for food-producing animals.
-
•
Economic interventions should be explored as a viable alternative to current bans on antibiotic usage in food-producing animals.
1. Introduction
Antimicrobial usage is a key contributor to rising antimicrobial resistance (AMR) in food-producing animals and human populations [1]. In particular, the usage of clinically important antimicrobials in food-producing animals has been linked to AMR in agricultural settings, which can spread to humans through foodborne, direct, or environmental transmission [[2], [3], [4], [5], [6]]. With an estimated 73% of all antimicrobials worldwide used in food-producing animals, an increased focus has been placed on controlling usage of veterinary antimicrobials to help preserve the efficacy of therapeutics for use in agriculture and human populations [7].
Interventions to target antimicrobial usage include bans or regulations on the prophylactic usage of antibiotics in agriculture. Such interventions were initiated in the EU in the 1990s, and with current WHO guidelines recommending the restriction of antimicrobials in growth promotion roles [[8], [9], [10]]. Bans on individual compounds and mode of application of veterinary antibiotics have also been successful, with literature suggesting reductions to resistance in clinically important Enterococcus spp. in broiler chickens and cattle. [4,11,12].
Market based instruments (MBIs) are an alternative to bans on antibiotic usage and include interventions such as taxation. MBIs have the benefit of allowing flexible compliance compared to bans, reducing the selective pressure for AMR by disincentivising usage, and generating income [7,[13], [14], [15]]. To date, antibiotic taxation schemes have only been implemented in a limited number of countries. Examples include a 2014 Belgian tax on veterinary antibiotics, based on the quantity of active ingredient sold and a Danish tax on critical care antibiotics [16,17]. This type of differentiated tax was originally implemented in pesticides and have also been effective, resulting in more conservative usage of toxic pesticides [[18], [19], [20]]. If applied in the context of antibiotics, these types of differentiated taxes could be used to reduce the usage/resistance of antibiotics with a high level of clinical importance.
Understanding and quantifying the impact of interventions, such as taxation, is complex due to the unintuitive nature of AMR dynamics [21]. However, epidemiological models can be used to better understand the non-linear dynamics of AMR and the impact of interventions [22]. Previous attempts to quantify the impact of taxation on antibiotic usage and AMR have extrapolated from economic analyses using metrics such as price elasticity of demand (PED) [7]. However, no model to date has explored how the price elasticities of antibiotics used in food-producing animals can be linked to the epidemiology of AMR, and how the non-linear dynamics of AMR might respond to the introduction of taxation.
In this work, synthetic price elasticities of demand are combined with an epidemiological model. We compare the effectiveness of different taxation strategies on the altering the price and usage of antibiotics, and subsequently on the prevalence of AMR, the number of overall infections caused by both drug-sensitive and resistant pathogens, and the maintenance of a portfolio of available antibiotics under a 25% resistance threshold. We explore multiple taxation strategies: flat taxation applied uniformly across all antibiotic classes, targeting single antibiotic classes and scaling taxation for each antibiotic class proportional to their relative level of resistance in food-producing animals (differential taxation). The effectiveness of taxation is compared with bans on usage of specific classes of antibiotics.
2. Materials and methods
2.1. Epidemiological model
The proportion of food-producing animals uninfected (U), infectious with wild-type bacteria (WT), bacteria resistant to one drug (R1, R2, R3), two drugs (R12, R13, R23) or three drugs (R123) was modelled using a deterministic model (Fig. 1). Uninfected food-producing animals are colonised/infected at a per-capita transmission rate (β) and enter the uninfecteds through birth rate λ, and leave through mortality at the same rate to ensure a constant population size. Transmission-related fitness costs of resistance were also modelled, reducing the efficacy of transmission for individuals infected with antibiotic-resistant strains (c1, c2, c3, c12, c13, c23, c123). Fitness costs had the impact of driving heterogeneity in transmission across the three modelled drug resistance classes. Recovery from WT, singly resistant, doubly resistant and triply resistant infections were modelled as rates rWT, rr, rrr, rrrr respectively and were dependent on the extent of antibiotic usage of the three modelled antibiotic classes.
Fig. 1.
Transmission and generation of multi-drug resistance (MDR) in a population of food-producing animals exposed to three hypothetical antibiotic classes. Uninfected (U), Wild-Type (WT), Resistant to Class 1 (R1), Resistant to Class 2 (R2), Resistant to Class 3 (R3), Resistant to Class 1 + 2 (R12), Resistant to Class 1 + 3 (R13), Resistant to Class 2 + 3 (R23) and Resistant to Class 1 + 2 + 3 (R123). The full set of ordinary differential equations relating to the model can be found in the Supplementary Material.
Antibiotic usage was described as the proportion of food-producing animals exposed to one of three antibiotic classes (σ1, σ2, σ3). The impact of antibiotic exposure is two-fold: 1) a therapeutic effect resulting in an increased rate of recovery (rt), with probability 1-ρ [23] and 2) a probability of treatment failure resulting in gain of resistance (ρ). Treatment failure occurs at rate ηwt, ηrr and ηrrr for individuals colonised with singly, doubly or triply resistant strains.
Treatment failure and generation of resistance was assumed to occur in a stepwise fashion and can be considered analogous to antibiotic usage acting as a selection pressure, allowing for the proliferation of an implicitly assumed, small, non-infectious quantity of resistant bacteria. Reversion from resistance back to WT infection was assumed to only occur in infected food-producing animals not exposed to antibiotics (1-σ1 + σ2 + σ3), with a lack of antibiotic pressure allowing for fitter WT bacteria to outcompete resistant strains [24].
2.2. Baseline model fitting
The three antibiotic classes were fitted as a baseline scenario using an approximate Bayesian computation sequential Monte-Carlo (ABC-SMC) to ensure high (40%), medium (25%) and low (10%) levels of resistance respectively for the three antibiotics modelled (Supplementary Material) [25]. The aggregated dynamics of resistance to the three antibiotic classes were tracked in the model, defined as R1, R2 and R3, and defined as the sum of the population resistant to each antibiotic class (i.e. R1 = R1 + R12 + R13 + R123).
2.3. Taxation
Taxation was described as % alterations in the price of each antibiotic class, adapting the price elasticity of demand (PED) formula , to model a % change in the proportion of the population using a specific antibiotic (σ1, σ2, σ3).
A synthetic demand matrix described the effect of PEDs, with the diagonal and non-diagonal elements representing the own-PED for each antibiotic and cross-PED respectively. The cross-PED can be interpreted as the impact of taxation of antibiotic classes on the usage of other antibiotics. We use a case study assigning first (class 1), second (class 2) and last-line antibiotic (class 3) identities to the three antibiotic classes, with the range of elasticity values obtained from an estimate of the distribution of own price elasticities of demand of veterinary antibiotics (mean values of −1.75 to −0.68) [7].
| (1.1) |
Antibiotics with “first-line” identities were assumed to have higher elasticities due to the availability of alternative antibiotics, and that the elasticities of successive antibiotic classes decrease due to having fewer alternatives. The cross-PED was higher for antibiotics next in “priority” to the taxed antibiotic (e.g. antibiotic class 1 to 2), with an assumption that veterinarians are more likely to switch to the antibiotic next in therapeutic “importance”.
2.4. Interventions
Three taxation strategies were modelled, fixed at a baseline rate of 50% (increase in price of 50%) introduced after the prevalence of resistance reaches an endemic equilibrium (t = 8.22 years or 3000 days): 1) Taxation applied across all antibiotics (Flat Tax; FT), 2) single taxation to highest, medium and lowest resistance antibiotic classes (ST) or 3) a differential taxation scheme (DT).
The principle of differential taxation is that taxation is scaled at intervals or “rounds” (every 3 years at baseline) relative to the prevalence of resistance in the taxed antibiotic class (Fig. S1–2). As an example, assuming an 0.8, 0.5 and 0.25 resistance prevalence across the three antibiotic classes, changes to the taxation rate would be: 1.6× for class 1 (0.8/0.5), 1× for class 2 (0.5/0.5), and 0.5× for class 3 (0.25/0.5).
Taxation strategies were compared against bans on antibiotic usage to identify if similar impacts can be achieved when compared to the status quo of antibiotic usage interventions, in highest, medium, or lowest resistance antibiotic classes. Note that all non-differential taxation interventions were constant throughout the intervention period.
2.5. Performance criteria
To compare the efficacy of the intervention strategies, we considered three criteria relevant to AMR in food-producing animals: 1) maximising decreases to average resistance across all antibiotics, 2) preventing increases to overall infections and animal disease due to a loss in antibiotic pressure (due to changes in usage) and 3) maximising the average number of available antibiotics (defined at a baseline prevalence of resistance <25%) over the course of the modelled intervention period (t = 10,000 days or 20 years). The first two criteria were calculated relative to the change in overall antibiotic usage (Eq. 1.2–1.3).
| (1.2) |
| (1.3) |
These relative performance criteria identify “efficient” interventions that have the greatest reductions in AMR for a given change in antibiotic usage (standardised at total antibiotic curtailment; σ1 + σ2 + σ3 = 1 to 0). These relative criteria also enable direct comparisons between interventions.
2.6. Uncertainty analysis
The proportion of times that each intervention was the “best-performing” strategy for each criterion was identified by running the interventions on n = 1000 biologically plausible parameter sets generated through Monte-Carlo sampling of parameters. Parameter ranges were centred around the baseline values obtained through ABC-SMC model fitting (Table 1).
Table 1.
Parameter ranges used for uncertainty analysis.
| Parameter | Description | Parameter Range | Baseline Values | References |
|---|---|---|---|---|
| λ | Birth/death rate in food-producing animals | 7300−1 - 36.5−1 days | 365−1 days | [26] |
| β | Rate of transmission between infected and uninfected food-producing animals | 0–10 | 4.919 | Fitted |
| σx | Proportion of the population using class X antibiotic | 0–1 | 0.25 | Assumed |
| rX | Rate of recovery for food-producing animals with X-type infection | 365−1 - 1−1 days | rwt = 1/12 rr = 1/10 rrr = 1/8 rrrr = 1/8 rt = 1/7 |
Assumed |
| ηwr1 | Rate of conversion from wild-type to antibiotic-resistant infection | 0.15–10 | 1.531 | Fitted |
| ηrw1 | Rate of reversion from antibiotic-resistant to wild-type infection | 0.006–0.6 | 0.062 | Fitted |
| ηrr1 | Rate of conversion from singly resistant to doubly resistant infection | 0.009–0.9 | 0.094 | Fitted |
| ηrrr1 | Rate of conversion from doubly resistant to triply resistant infection | 0.009–0.9 | 0.094 | Fitted |
| cX1 | Transmission-related fitness cost related to infection with resistance to class X antibiotic | 0.5–1 | c1 = 0.96 c2 = 0.90 c3 = 0.66 c12 = 0.63 c13 = 0.60 c23 = 0.59 c123 = 0.54 |
Fitted |
| ρ | Probability of treatment failure upon exposure to effective antibiotic | 0–1 | 0.05 | Assumed |
| Base Tax | Baseline taxation rate | 0–1 | 0.5 | Assumed |
Parameters were subject to the following inequality constraints c1 ≈ c2 ≈ c3 > c12 ≈ c13 ≈ c23 > c123, rwt > rr > rrr > rrrr > rt, (with n antibiotic classes) and ηWR > ηRW.
This metric was weighted by the probability of intervention failure, defined as an increase of both usage and resistance resulting from the intervention (Supplementary Material). The distribution of effect sizes for each intervention across each criterion was also assessed.
2.7. Scenario analyses
Scenario analyses were conducted to assess the robustness of the model to changes in model assumptions. This included alterations to the baseline taxation rate (10%, 25%, 75%, 90%), cross-elasticity of demand (0.4 or 0.6), varying the entire PED matrix (sampling the cross-PED from 0 to 1 and own-PED from −2 to 0), varying the threshold for an “effective antibiotic” (5%, 10%, 35%), the number of modelled antibiotics (two or four) and modelling specific food-producing animals (cattle λ = 365−1 days; broiler chicken λ = 42−1 days) and production systems (extensive β = 0.5; intensive β = 50).
2.8. Estimated yearly revenue from taxation
To identify the global annual revenue from the taxation of veterinary antibiotics used in food-producing animals (US$) for each taxation strategy, information was combined on: 1) model output on the % increases in price (taxation rate) over the 20-year intervention period for each intervention, and 2) the yearly market value of different antibiotic classes in food-producing animals.
To generate an estimate for the yearly market value of veterinary antibiotics, information on the sales and price of different antibiotic classes in food-producing animals was combined for each country globally. Details of the included antibiotic classes can be found in the Supplementary Material. Antibiotic price data from the US and China were used as proxies to represent high-income countries (HICs) and low/middle-income countries (LMICs) respectively. Pricing data was identified from online retailers and harmonised to US Dollars (US$) using a conversion rate from Chinese Yuan (Supplementary Material) [27,28]. The proxied price for HICs and LMICs was then multiplied by global antibiotic sales data for food-producing animals for each antibiotic class in every HIC and LMIC [29,30]. This enabled a determination of the yearly global revenue (US$) from each antibiotic class for all HICs and LMICs. Note that the total estimated revenue across each antibiotic class was scaled to match the estimated market value for veterinary antibiotics of 2.731 billion US$ [31].
Elasticity values were next assigned to each antibiotic class based on their respective level of resistance, classifying antibiotics as one of the three antibiotics modelled (1, 2 or 3) in the synthetic PED matrix (eq. 1.1). Prevalence data on resistance for each antibiotic class in Salmonella spp. and Campylobacter spp. was obtained from the 2021 NARMS report (US) and resistancebank.org (China) [32,33]. Higher resistance antibiotic classes were assumed to correspond to less critical antibiotics (group 1) (Supplementary Material).
3. Results
3.1. Efficacy of taxation schemes and bans
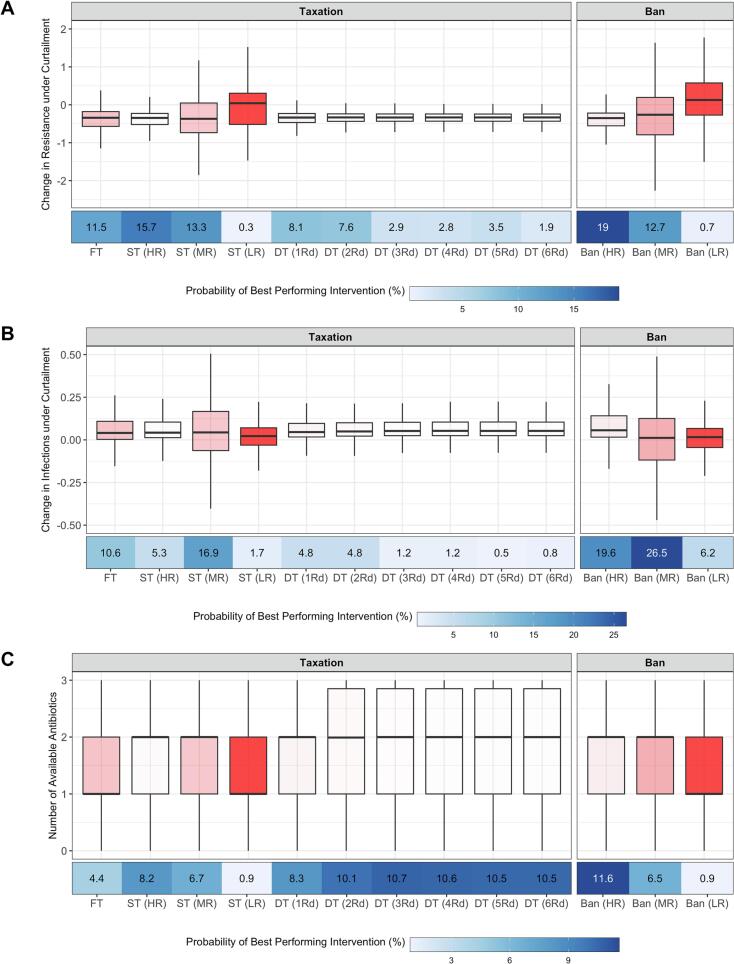
Changes to average resistance suggest that curtailment of veterinary antibiotic usage in food-producing animals from full usage (σ1 + σ2 + σ3 = 1) to no usage (σ1 + σ2 + σ3 = 0), would result in a median reduction of the proportion of resistant infections in food-producing animals ranging between 0.26 (Ban MR; 95% CI: −2.22, 1.63) to 0.37 (ST LR; 95% CI: −1.85, 1.17) (Fig. 2A) across the interventions. Increases in infection ranged between 0.01 (Ban MR; 95% CI: −0.46, 0.49) to 0.06 (Ban HR 95% CI: −0.17, 0.33) (Fig. 2B). Additionally, the average number of available antibiotics ranged across interventions from a median value of 1 to 2 available antibiotics (95% CI: 0, 3) (Fig. 2C). The exception to these ranges of effect sizes was with interventions to target the lowest levels of resistance (Ban LR: 0.12; 95% CI: −1.5, 1.77).
Fig. 2.
A) Changes to average resistance under total antibiotic curtailment, B) changes to overall infections under total antibiotic curtailment, C) Number of available antibiotics. FT = Flat Tax, ST = Single Tax, DT = Differential Tax, HR = High Resistance, MR = Medium Resistance and LR = Low Resistance. Multiple rounds of differential taxation were explored (1-6Rds). The intensity of box plot shading represents the proportion of runs resulting in increases to both usage and resistance, representing intervention failure (also used for weighting of intervention performance: 27.5%, 2.1%, 25.3%, 80.7%, 5.2%, 2.8%, 1%, 0.9%, 0.9%, 0.9%, 7.9%, 36% and 80.3%). Pairwise significance testing to compare distributions were included in the Supplementary Material.
However, bans (HR and MR) on antibiotic usage were identified as the “best” performing intervention according to the performance metric to minimise average resistance (19%; 95% CI: 17%, 22%), minimise increases in overall infection (26.5%; 95% CI: 24%, 29%) and maximise the average number of available antibiotics (11.6%; 95% CI: 11%, 12%) (Fig. 2).
Differential taxation was the most similar strategy to bans to maximise the average number of available antibiotics and with the smallest range of intervention failure rates (0.9% - 5.2%). Taken together across all three criteria, this suggests that taxation strategies can perform as effectively as bans on antibiotic usage for the range of parameters explored. To explore the similarity in effect size between taxation and bans, the taxation rate (HR) was incremented from 1% to 100%. A linear change in effect size to reduce resistance was observed, plateauing to a level comparable to bans at 60% taxation (HR: -0.36; 95% CI: −0.90, 0.13) (Fig. S5–6).
The results were found to be robust when alternative performance criteria were used: 1) absolute changes in resistance/infection, and 2) % changes to resistance/infections per % decrease in usage (Fig. S7–8). However, an exception was with an improved performance of single taxation to reduce resistance in the second alternative criteria. The results were also unchanged when the hierarchy of fitness costs assumption was removed (Fig. S9).
3.2. Scenario analyses
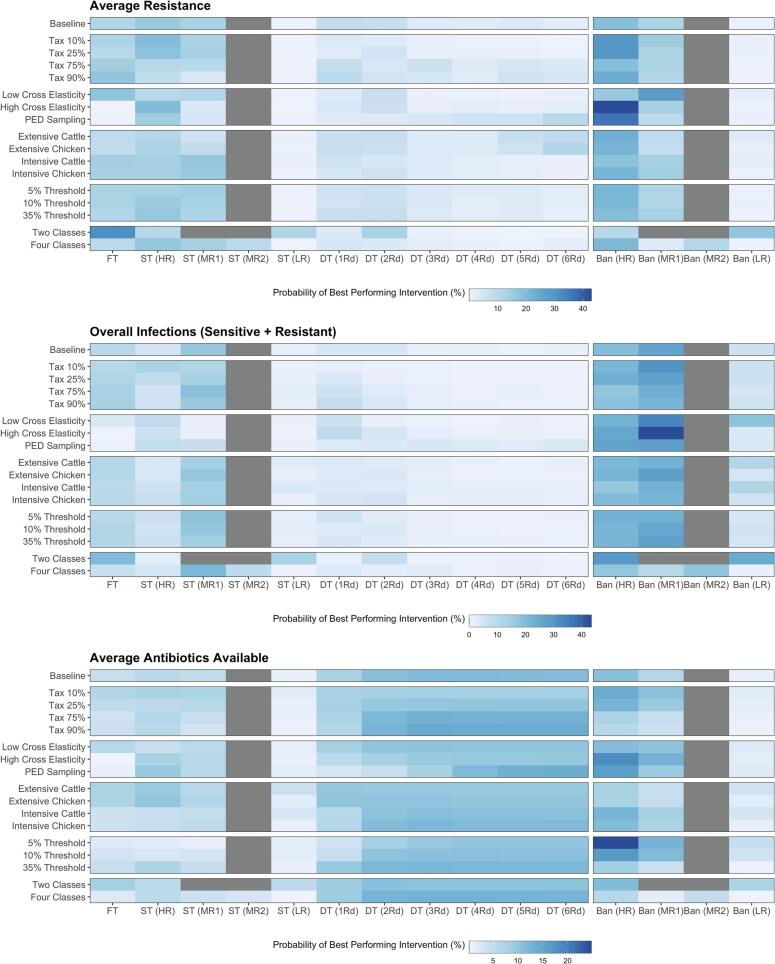
Alternative scenarios were used to explore the robustness of best performing interventions. These scenarios related to the extent of taxation (90%, 75%, 25% and 10%), altering the PED matrix (cross PED values of 0.4 or 0.6 or varying the matrix), the number of antibiotic classes (two vs four), a case study with cattle and broiler chicken production systems (β = 0.5 or 50; λ = 1/365−1 or 1/42−1 days) and the threshold for the resistance for which an antibiotic is considered “available” (5%, 10% and 35%) (Fig. 3).
Fig. 3.
Probability that each intervention performs best to minimise average resistance, minimise increases to overall infections, and maximise the number of available antibiotics across different scenarios. FT = Flat Tax, ST = Single Tax, DT = Differential Tax, HR = High Resistance, MR1 = 2nd Lowest Resistance, MR2 = 3rd Lowest Resistance, LR = Low Resistance. The baseline for rescaling for differential taxation in the scenarios with two and four antibiotic classes was calculated as the average of the two medium resistance classes (4 classes) or the average of the only two antibiotic classes (2 classes). Note that box plots with effect sizes for all scenarios can be found in the Supplementary Material (Fig. S11–26).
The qualitative pattern of best performing interventions was maintained across alternative scenarios, with bans on antibiotic usage maintained as the being the best performing strategy to reduce average resistance and overall infection (Fig. 3). The exception to this qualitative pattern was modelling two antibiotic classes, which increased the performance of taxation above bans on usage. Stricter thresholds for an available antibiotic, reducing the rate of taxation, varying the PED matrix and cross elasticity of demand also reduced the performance of differential taxation. The efficacy of flat taxation was also strongly impacted by alterations to the PED matrix. The use of extensive production reduced the probability of intervention failure and increased the average availability of effective antibiotics (Fig. S23–26).
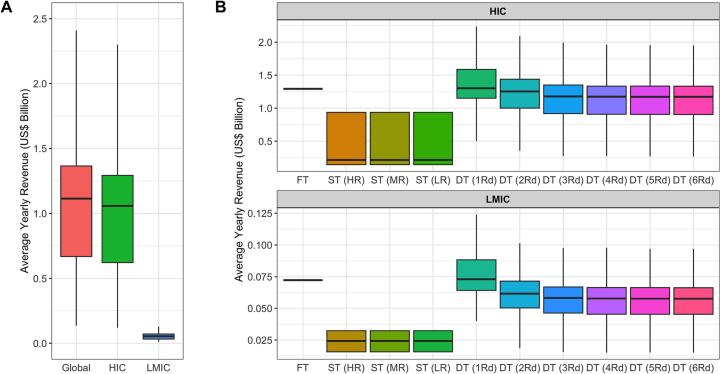
3.3. Estimated revenue from taxation
The median global yearly revenue averaged across all considered taxation strategies was $1.11 billion (95% CI: 0.13, 2.4) (Fig. 4A). Differential taxation ($1.3 billion; 95% CI: 0.5, 2.23) and flat taxation strategies ($1.29 billion; 95% CI: 1.29, 1.29) generated the largest median revenue across explored taxation strategies (Fig. 4B).
Fig. 4.
Projected revenue from taxation strategies for High-Income Countries (HICs), Lower-Middle Income Countries (LMICs). A) Aggregated median income for all countries, HICs and LMICs across all explored taxation strategies. B) Estimated revenue for each taxation strategy. FT = Flat Tax, ST = Single Tax, DT = Differential Tax, HR = High Resistance, MR = Medium Resistance, LR = Low Resistance.
The average yearly median revenue across all taxation strategies was higher in HICs ($1.06 billion; 95% CI: 0.11, 2.3) when compared to LMICs ($0.05 billion; 95% CI: 0.01, 0.12), attributable to the higher average price of antibiotics in the former case study (Supplementary Material). This analysis was also explored with alternative tax rates of 25% and 75%, with median global yearly revenue of $0.61 (95% CI: 0.06, 1.2) and $1.52 (95% CI: 0.18, 3.86) billion respectively (Fig. S30–31).
4. Discussion
Taxation strategies were found to have similar median effect sizes to outright bans on antibiotic usage in food-producing animals to maximise reductions to resistance, minimise increases in infections and maximise the average number of available antibiotics. Additionally, taxation could generate a global median annual revenue of $1.11 billion under a taxation rate of 50% that could be reinvested into antibiotic development or agricultural biosecurity. However, bans on antibiotic usage could more frequently achieve these effect sizes across the three criteria when compared to taxation for the explored parameter space.
Bans on antibiotic usage were identified as the best performing intervention across all three criteria. This can be attributable to the low variance and having the largest median effect size when compared across all modelled strategies. This adds support to the current and historical implementation of bans to control AMR in food-producing animals [34]. However, differential taxation, flat taxation and single taxation to high resistance (HR) classes also resulted in comparable variance and median effect sizes to bans, suggesting that taxation can be a plausible alternative to implemented bans on usage.
Market based interventions (MBIs) such as taxation and its near equivalent of a cap and tradeable permit scheme also have benefits over bans, allowing for flexibility in the usage of antibiotics, with bans being punitive of all users irrespective of their relative cost of abatements [15]. MBIs also generate yearly revenue, with ∼1 billion US$ estimated in this study. This should be placed into context with the estimated 1 to 2 billion US$ development cost for a novel antibiotic and the 540 billion US$ global support to agricultural producers, which could help improve biosecurity and reduce reliance on antibiotics used in food-producing animals [7,35,36]. In more budget restricted LMICs, the additional revenue could be directed for non-agricultural purposes with indirect benefits for farmers [37]. The introduction and coordination of MBIs can also be facilitated by National Action Plans (NAPs), with taxation collected by retailers and sent to respective government agencies [18,38].
Use of extensive production systems with lower transmission will also impact the efficacy of interventions, increasing the average availability of effective antibiotics and reducing intervention failure (Fig. 3). Mode of application may also impact patterns of usage, with prescription-based EU usage of antibiotics for food-producing animals likely reducing elasticity of antibiotics due to a limited access to alternatives [39]. Future understanding of the impact of economic interventions would benefit greatly from more accurate insight into the response of antibiotic usage to changing market conditions and production systems.
However, the main advantage of bans over taxation is the certainty in reductions to usage. In contrast, the efficacy of MBIs will be sensitive to uncertainty in market dynamics and some users may simply opt to pay a tax rather than altering consumption patterns [19]. There are also questions about the incidence of the burden of taxation, which will ultimately fall on the consumer, and the potential impacts on food security [40]. However, it is worth noting that given the punitive nature of bans, the harms of bans while reducing usage will be greater than for taxation. MBIs such a permit trading scheme can alternatively guarantee changes in usage through caps on usage, but carry uncertainty in the generation of revenue [15]. Future assessments of the viability of MBIs must therefore consider criteria other than the epidemiological outcomes explored by this study.
Differential taxation was identified as an effective strategy in this study, dynamically controlling resistance across the portfolio of antibiotics by promoting periods of high and low resistance [23,41,42]. The targeted nature of differential taxation is also what promotes the low level of intervention failure, preventing large compensatory increases in resistance. Differential taxation also resulted in the greatest certainty in effect size across considered interventions, which may prove to be beneficial for the risk-averse nature of agricultural policy making [43]. These unique benefits of differential taxation, combined with the similar median effect size to bans, suggests that differential taxation should be explored as a tool to ensure the availability of veterinary antibiotics.
However, the cyclical nature of differential taxation may mean antibiotics critical for food-producing animals such as glycopeptides and streptogramins undergo periods of high resistance [44]. Differential taxation will also require periodical recalibration of taxation, which might prove difficult for implementation, with literature citing stability and ease of implementation being critical for effective taxation [18,43]. Although beyond the scope of this study, future models could explore additional layers of taxation on “last-line” antibiotics, similar to contemporary Danish or Belgian schemes [16,17]. Implementation of differential taxation will require improvements to current AMR surveillance, so that taxation can be accurately calibrated based on the prevalence in each antibiotic class.
Despite HICs only making up 20.4% of global sales of veterinary antibiotics, HICs were estimated to contribute the majority of global revenue from taxation. However, the variation surrounding this estimate suggests scenarios where revenue can be twice higher than the median, or close to levels observed in LMICs. Risk-averse policy makers may opt for flat taxation, with little variation in revenue expected if this is the primary goal. The result of this analysis also introduces a series of ethical questions: is the unequal contribution from HICs fair, considering the historical usage of veterinary antibiotics and higher GDP in HICs? Additionally, what would be the implications of these taxes be on small-holding farmers in LMICs? Could a scheme be introduced to compensate farmers in LMICs for potential losses in productivity, or to implement taxation in HICs only?
Two scenarios demonstrated relatively higher rates of intervention failure: 1) high cross elasticity of demand, or 2) targeting the lowest resistance antibiotic class. Both can be attributed to the impact of compensatory increases in usage beyond what is being reduced through the intervention. Implementation of future economic interventions must avoid scenarios which lead to this increased risk of intervention failure, which has been identified in the implementation of pesticide taxation [19].
By integrating an epidemiological model with the price elasticity of demand, this study provides a first quantitative exploration into the viability and efficacy of taxation as a potential strategy to tackle AMR in food animals. We highlight taxation, as a potential tool for AMR control in agriculture, which should be explored through further modelling work. However, this must also be done in tandem with improvements to surveillance and an increased understanding of the elasticity of antibiotics for use in food animals. It is vital that we continue to explore tools and strategies that can effectively reduce AMR to tackle the ongoing AMR crisis in both food animals and in humans.
Authors' contributions
ALKM participated in the study design, carried out model analysis and drafted the manuscript. TPVB participated in the study design and provided feedback on manuscript drafts. DM provided feedback on manuscript drafts.
Funding
ALKM and TPVB were supported by the Swiss National Science Foundation Eccellenza Fellowship (No 181248) and the Branco Weiss Foundation. DM wishes to acknowledge UKRI funding under grant numbers BB/W018152/1, BB/T004436/1.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Acknowledgements
We thank colleagues from the Health Geography and Policy group and Professor Sebastian Bonhoeffer at ETH Zürich for their helpful comments, feedback and discussions during the drafting of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100650.
Appendix A. Supplementary data
Supplementary material (text and figures)
Data availability
I have attached a GitHub link in the “attach data” step Github Repository (Original data)
References
- 1.Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365(6459) doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 2.Woolhouse M., Ward M., Van Bunnik B., Farrar J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B: Biol. Sci. 2015;370(1670) doi: 10.1098/rstb.2014.0083. 20140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London, UK. 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf Available from:
- 4.Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet. Health. 2017;1(8) doi: 10.1016/S2542-5196(17)30141-9. e316-e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veterinary Medicines Directorate . Veterinary Medicines Directorate; New Haw, Addlestone: 2019. UK One Health Report - Joint Report on Antibiotic Use and Antibiotic Resistance, 2013–2017.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/921039/Ted_Final_version__1318703-v45-One_Health_Report_2019_FINAL-accessible.pdf Available from: [Google Scholar]
- 6.McEwen S.A., Collignon P.J. Antimicrobial resistance: a one health perspective. Microbiol. Spectr. 2018;6(2):6–10. doi: 10.1128/microbiolspec.arba-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Boeckel T.P., Glennon E.E., Chen D., Gilbert M., Robinson T.P., Grenfell B.T., et al. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Commission . European Commission; Brussels: 2020. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System.https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en Available from: [Google Scholar]
- 9.Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC, Regulation (EU) 2019/6. 2019. [Google Scholar]
- 10.Aidara-Kane A., Angulo F.J., Conly J.M., Minato Y., Silbergeld E.K., McEwen S.A., et al. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 2018;7:1–8. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarestrup F.M. Get pigs off antibiotics. Nature. 2012;486(7404):465–466. doi: 10.1038/486465a. [DOI] [PubMed] [Google Scholar]
- 12.Aarestrup F.M., Seyfarth A.M., Emborg H—D, Pedersen K, Hendriksen R, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 2001;45(7):2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollis A., Ahmed Z. Preserving antibiotics, rationally. N. Engl. J. Med. 2013;369(26):2474–2476. doi: 10.1056/NEJMp1311479. [DOI] [PubMed] [Google Scholar]
- 14.Berendse F. Add a tax to the EU agricultural policy. Nature. 2017;543(7645):315. doi: 10.1038/543315a. [DOI] [PubMed] [Google Scholar]
- 15.Helfand G.E., Berck P., Maull T. In: Handbook of Environmental Economics. Mäler K.-G., Vincent J.R., editors. Elsevier; 1: 2003. Chapter 6 - The Theory of Pollution Policy; pp. 249–303. [Google Scholar]
- 16.Agence fédérale des médicaments et des produits de santé Taxe sur les antibiotiques. Agence fédérale des médicaments et des produits de santé. 2016. https://www.afmps.be/fr/veterinaire/medicaments/medicaments/bon_usage/Antibiotiques_problematique_resistance/taxe_sur_les [updated 16/12/20. Available from:
- 17.Statens Serum Institut . Statens Serum Institut, National Food Institute, Technical University of Denmark; Lyngby: 2019. DANMAP 2018 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark.https://www.danmap.org/reports/2018 Available from: [Google Scholar]
- 18.Böcker T., Finger R. European pesticide tax schemes in comparison: an analysis of experiences and developments. Sustainability. 2016;8(4):378. [Google Scholar]
- 19.OECD . 2020. Taxation in Agriculture. [Google Scholar]
- 20.Tiley S.M., Fergusson J., Ferguson J., Macdonald J., Doherty P. Active promotion of antibiotic guidelines: an intensive program. Commun. Dis. Intell. Q. Rep. 2003;27(2003) doi: 10.33321/cdi.2003.27.15. [DOI] [PubMed] [Google Scholar]
- 21.Spicknall I.H., Foxman B., Marrs C.F., Eisenberg J.N. A modelling framework for the evolution and spread of antibiotic resistance: literature review and model categorisation. Am. J. Epidemiol. 2013;178(4):508–520. doi: 10.1093/aje/kwt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith D.L., Harris A.D., Johnson J.A., Silbergeld E.K., Morris J.G., Jr. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc. Natl. Acad. Sci. U. S. A. 2002;99(9):6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonhoeffer S., Lipsitch M., Levin B.R. Evaluating treatment protocols to prevent antibiotic resistance. Proc. Natl. Acad. Sci. 1997;94(22):12106–12111. doi: 10.1073/pnas.94.22.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies N.G., Flasche S., Jit M., Atkins K.E. Within-host dynamics shape antibiotic resistance in commensal bacteria. Nat. Ecol. Evol. 2019;3(3):440–449. doi: 10.1038/s41559-018-0786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toni T., Welch D., Strelkowa N., Ipsen A., Stumpf M.P. Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J. R. Soc. Interface. 2009;6(31):187–202. doi: 10.1098/rsif.2008.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RSPCA Production Process - BEEF CATTLE. 2023. https://www.rspca.org.au/sites/default/files/responsible-sourcing/documents/Production-Process-Beef-Cattle.pdf Available from:
- 27.Food & Drug Administration . FDA; Maryland: 2022. 2021 Summary Report On Antimicrobials Sold or Distributed for Use in Food-Producing Animals.https://www.fda.gov/media/163739/download Available from: [Google Scholar]
- 28.Ministry of Agriculture and Rural Affairs of the People’’s Republic of China . Ministry of Agriculture and Rural Affairs of the People’s Republic of China; Beijing: 2020. Official Veterinary Bulletin.http://www.moa.gov.cn/gk/sygb/ Available from: [Google Scholar]
- 29.The World Bank . The World Bank; Washington, D.C., United States: 2021. World Bank Country and Lending Groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [cited 2023 06/01/2023]. Available from: [Google Scholar]
- 30.Mulchandani R., Wang Y., Gilbert M., Van Boeckel T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health. 2023;3(2) doi: 10.1371/journal.pgph.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SCSP Global . Stonehaven Consulting; Frauenfeld, Switzerland: 2022. Farm Animal Antibiotics Market 2022: Animal Health Market Analysis. Available. [Google Scholar]
- 32.Food & Drug Administration . U.S. Department of Health and Human Services; Rockville, MD: 2023. NARMS Now.https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/narms-now-integrated-data Available from: [Google Scholar]
- 33.Criscuolo N.G., Pires J., Zhao C., Van Boeckel T.P., resistancebank. Org, an open-access repository for surveys of antimicrobial resistance in animals. Sci. Data. 2021;8(1):1–10. doi: 10.1038/s41597-021-00978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson J.M., Chiller T.M., Powers J.H., Angulo F.J. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin. Infect. Dis. 2007;44(7):977–980. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- 35.FAO, UNDP, UNEP . FAO; Rome: 2021. A Multi-Billion-Dollar Opportunity – Repurposing Agricultural Support to Transform Food Systems. Available. [Google Scholar]
- 36.Laxminarayan R. Antibiotic effectiveness: balancing conservation against innovation. Science. 2014;345(6202):1299–1301. doi: 10.1126/science.1254163. [DOI] [PubMed] [Google Scholar]
- 37.OECD . OECD; Paris: 2022. Tax Policy Reforms in Low-and Middle-Income Countries: Policy brief.www.oecd.org/tax/tax-policy/tax-policy-reforms-in-low-and-middle-income-countries-policy-brief.htm Available from: [Google Scholar]
- 38.Möhring N., Ingold K., Kudsk P., Martin-Laurent F., Niggli U., Siegrist M., et al. Pathways for advancing pesticide policies. Nat. Food. 2020;1(9):535–540. doi: 10.1038/s43016-020-00141-4. [DOI] [PubMed] [Google Scholar]
- 39.Yeung K., Basu A., Hansen R.N., Sullivan S.D. Price elasticities of pharmaceuticals in a value based-formulary setting. Health Econ. 2018;27(11):1788–1804. doi: 10.1002/hec.3801. [DOI] [PubMed] [Google Scholar]
- 40.Weitzman M.L. Prices vs. quantities. Rev. Econ. Stud. 1974;41(4):477–491. [Google Scholar]
- 41.Bergstrom C.T., Lo M., Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc. Natl. Acad. Sci. 2004;101(36):13285–13290. doi: 10.1073/pnas.0402298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tepekule B., Uecker H., Derungs I., Frenoy A., Bonhoeffer S. Modeling antibiotic treatment in hospitals: a systematic approach shows benefits of combination therapy over cycling, mixing, and mono-drug therapies. PLoS Comput. Biol. 2017;13(9) doi: 10.1371/journal.pcbi.1005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chèze B., David M., Martinet V. Understanding farmers’ reluctance to reduce pesticide use: A choice experiment. Ecol. Econ. 2020;167 [Google Scholar]
- 44.AMEG, CMVP, CHMP . European Medicines Agency; Amsterdam: 2019. Categorisation of antibiotics in the European Union - Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals.https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material (text and figures)
Data Availability Statement
Data availability
I have attached a GitHub link in the “attach data” step Github Repository (Original data)