Abstract
Ethnopharmacological relevance
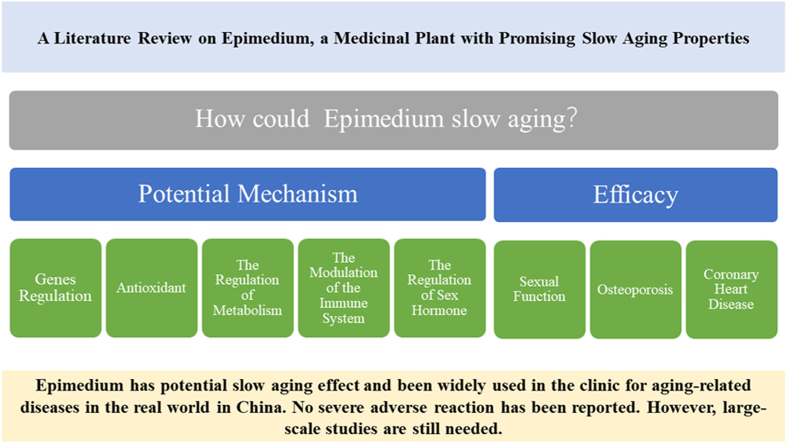
Aging is related to many factors, such as genes, oxidative damage, metabolic abnormalities, immune regulation and sex hormones. This article reviews the pharmacological mechanism of Epimedium on slow aging from six aspects: gene regulation, antioxidant, the regulation of metabolism, the modulation of the immune system, the regulation of sex hormone, and clinical efficacy.
Aim of the studyThrough literature review, to discover the potential pharmacological mechanism of Epimedium for slow aging.
Materials and methods
We reviewed the literature on the applications of Epimedium in multiple systems and the potential underlying mechanisms with systematic and comprehensive illustrations. The review includes the following aspects: gene regulation, antioxidant, the regulation of metabolism, the modulation of the immune system, the regulation of sex hormone, clinical efficacy and safety.
Results
The slow aging active components of Epimedium may be flavonoids, such as Epimedins A, B, C and icariin The slow aging effect of Epimedium may be related to gene regulation, antioxidant, the regulation of metabolism, the modulation of the immune system, and the regulation of sex hormone. No severe adverse reaction has been reported.
Conclusions
Epimedium has potential slow aging effect and been widely used in the clinic for aging-related diseases in the real world in China; however, large-scale studies are still needed.
Keywords: Epimedium1, Aging2, Antioxidant3, Mechanism4, Metabolism5
Graphical abstract
Highlights
-
•
The slow aging mechanism of Epimedium was reviewed for the first time.
-
•
According to the theory of traditional Chinese medicine, the sexual dysfunction caused by kidney qi deficiency is also included in the aging mechanism.
-
•
Both pharmacological mechanism and clinical efficacy are considered.
1. Introduction
Aging is a complex and multifactorial process characterized by an increased risk of adverse health outcomes [1] and is becoming a predominant health problem worldwide. It has a significant impact on individual quality of life and public health strategies. Traditional Chinese medicines (TCMs) are essential parts of complementary and alternative medicine and may have a promising future in the slow aging sphere.
From the TCM view, Epimedium (Berberidaceae) has rejuvenation and energetic functions and has been commonly used in many traditional formulas for centuries via “nourishing the kidney and reinforcing the Yang” [2,3]. Studies have shown that Epimedium possesses multispectral therapeutic activities, including slow aging, preventing osteoporosis, boosting immunity, anti-depression, anti-myocardial ischemia, treating erectile dysfunction, and antineoplastic effects [[4], [5], [6]]. It has also been applied with other Chinese herbal medicines to treat various age-related diseases, such as cardiovascular diseases, sexual dysfunction, immune deficiency, and menstrual disorder [[7], [8], [9]]. Although in modern medical theory, aging and sexual function are not directly related, in traditional Chinese medicine theory, aging is closely associated with kidney qi weakness. One of the main manifestations of kidney qi weakness is sexual dysfunction. Epimedium (also named Barrenwort or Rowdy Lamb Herb or Horny Goat Weed, in Chinese as Yin Yang Huo, Yang He Ye or Xian Ling Pi (shown in Fig. 1), is a commonly used herbal in TCMs or herbal tea. Epimedium is a genus of perennial woodland herbs, and widely distributed under forests, in thickets or slopeswith an altitude of 650–3000 m.Epimedium is the largest herbaceous genus of Berberidaceae. Epimedium and preliminarily recognized that the genus comprises about 62 species. As an Old World genus, Epimedium is distributed disjunctively and very unevenly in woodlands or scrubs in the Mediterranean region, western Asia and eastern Asia. Five species are distributed in Algeria and Caucasus, six species distributed in Japan, Korea, north-eastern China and Far Eastern Russia, about 51 species in central southeastern China.China is the diversity centre and distribution centre for Epimedium [10].Herbal Epimedium is prepared from the dried aerial parts of Epimedium wushanense T.S. Ying. (E. wushanense), Epimedium sagittatum Maxim (E. sagittatum), Epimedium brevicornum Maxim (E. brevicornum), Epimedium koreanum Nakai (E. koreanum) and Epimedium pubescens Maxim (E. pubescens) in Sichuan Province, Shanxi Province, Liaoning Province, and Gansu Province in China [11]. The therapeutic processing procedure of herbal Epimedium includes stir-frying with suet, wine, fire moxibustion, salt, butter, etc. Of them, stir-frying with suet is the most common (shown in Fig. 2) [12]. Epimedium is mainly composed of a diversity of flavonoids, where Epimedium flavonoids (EFs) are the major flavonoids, with icariin (ICA, molecular Formula C33H40O15) as the active monomer [13]. Prenyl flavonoids, Epimedium polysaccharides, alkaloids, phytosterols, terpenoids, chlorogenic acid, and other bioactive components are also contained in Epimedium [14,15].EFs and their derivatives are significant components of the genus Epimedium. In 17 Epimedium species, more than 141 flavonoids have been found such as chalcone, flavone, flavonol, flavonol glycoside and flavanone [16]. From the genus Epimedium, 31 lignans and the corresponding glycosides were identified, 12 ionones and their derivatives have been isolated, and only 6 phenethyl alcohol glycosides were identified up to now. Other compounds along with the above mentioned constituents like xanthones, aldehydes, acids, and alkaloids were also isolated from Epimedium plants. Epimedins A, B, C, and ICA makes up 52 % of the total flavonoids and above of herbal Epimedium, which are considered as major bioactive components [16] (see Fig. 3).
Fig. 1.
Epimedium sent forth from the dried aerial parts of Epimedium.
Fig. 2.
Yangheye is used as a traditional formulas herb after stir-frying with suet.
Fig. 3.
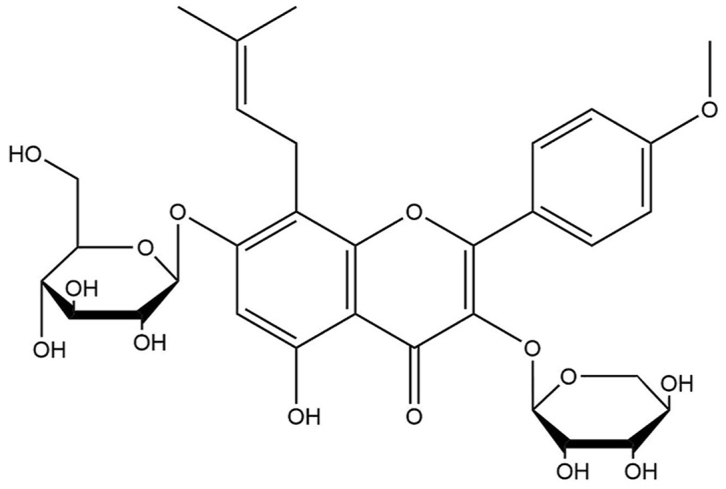
Epimedium was mainly composed of alkaloids and flavonoids which majority component was Epimedium flavonoids (EF), and the active monomers was Icarriin (ICA, Molecular formula C33H40O15).
2. Potential mechanism of epimedium in slow-aging
2.1. Genes regulation
2.1.1. Aging-related genes
Current studies have shown that Epimedium has the effect of slow aging by reversing the expression of aging-related genes. Huang [17] found that an age-dependent pattern was shown in gene expression of 199 genesd, in which most of them were reversed by Epimedium. In neural network model, the output showed that Epimedium made the transcriptomics of 24-months-old rats 8–13 months old. Among the 1885 peaks detected, 17 metabolites were identified to have significant age-dependent changes, and intervention of Epimedium reset metabolites level to a younger level (18-month). Moreover, p16 gene mRNA expression increased in aging humans, and Epimedium might inhibit expression of p16 gene to delay cells aging [18]. The degree of cell aging might be reduced by inhibition of p16 expression of DNA repair capacity, and that life can be prolonged. The above studies have shown that Epimediummay have the ability to reverse aging by regulating aging-related genes and their expression.
2.1.2. Longevity related genes
The study suggested that the regulation of longevity gene expression significantly slowed the aging process and reduced the occurrence and development of many diseases [19]. Liu [20] found that EF could increasenuclear factor kappa-B (NF-κB) to a moderate extent by way of IKK/IκB/Rel/NF-κB and that its related signaling pathway was through the regulation of IκBε and IκBα in aged rats. They assigned 60 Sprague–Dawley (SD) rats into 6 groups as follows: 3-day (d), 4-month (m), 10 m, 18 m, 27 m, and 27 m + EF. The results showed the mean level of NF-κB signal transduction kinase-related mRNA expression decreased with aging in rat splenic lymphocytes. At 3 days after birth, the highest expression was observed, and the lowest level was observed at 18 months or 27 months afterwards. With the intervention of EF, the expression level in the aged rats was raised to the 10–18-month level.
Furthermore, the results confirmed the effectiveness of EF in postponing aging. Chen [21] found that the lymphocyte apoptosis percentage showed a significant difference between the older age group and the young age group, comparing the EF-treated group and the older age group (P < 0.01). Compared with the young group, in the older age group, 116 genes were upregulated, and 215 were downregulated. Compared with that in the old group, in the EF-treated group, 447 genes were upregulated and 456 were downregulated, which involved cell apoptosis and cell proliferation regulation. The role of EF is to reverse the abnormal changes in gene expression with opposite functions, such as apoptosis promotion and inhibition and proliferation enhancement and antagonization of genes, to rebuild advantageous gene expression equilibrium, or to reconstruct immune homeostasis in aged individuals. In summary, Epimedium and its active ingredients regulate longevity gene expression and reverse favorable gene expression for slow aging.
2.1.3. Anti- DNA damage
ICA is the primary pharmacologically active chemical constituent of Epimedium. Many studies have indicated the antioxidative effects of ICA on β-amyloid-mediated neurotoxicity, DNA damage, and vein endothelial cell oxidative injury [22]. Compared with the aging model group, ICA significantly increased the relative protein expression levels of NQO1, HO-1, and Nrf2 in the testis. In parallel, the relative protein expression levels of γ-H2AX, p-p53, and p21 were significantly decreased. ICA attenuates DNA damage in testicular germ cells of natural aging rats, which may be associated with the activation of the Nrf2/HO-1 signaling pathway [23]. Zhao's research found that total flavone of Epimedium (TFE) decreased expression levels of γH2AX in spermatogonia and primary spermatocytes and γH2AX focal formation, and had downregulation of 8-OHdG levels at the same time [24]. In addition to that, TFE inhibited expression levels of p-P53/p21 and chk1/chk2. With p53-dependent pathway, TFE reduces oxidative DNA damage effectively in the testes of aging rats. Chen found that TFE can significantly antagonize the DNA damage of mouse bone marrow cells induced by mitomycin C (MMC), which is characterized by a decrease in comet cell percentage and a shortening of comet tail length and has a significant dose-dependent relationship. TFE has an excellent protective effect on DNA damage in mouse bone marrow lymphocytes induced by MMC [25].
2.2. Antioxidant
2.2.1. Anti-free radical
In the 1950s, Harman first proposed the free radical theory of aging [26], in which age-associated functional losses are regarded as the accumulation of oxidative damage to macromolecules (lipids, DNA, and proteins) by reactive oxygen and nitrogen species produced by several endogenous and exogenous processes [27]. Excessive accumulation of free radicals in the body could cause cell damage and tissue and organ disorders, which would ultimately lead to aging. Epimedium can affect aging with many mechanisms, particularly by regulating endocrine and immune systems and improving metabolism and organ function. Furthermore, EFs are able to reduce liver oxygen levels in an isolated culture of hepatic tissue. Researchers have found that Epimedium can eradicate free radicals and decrease their reaction activity. Zhao [28] indicated that the phenolic compounds from Epimedium possessed significant antioxidant activity. The experiment indicated that Epimedium remarkably activated superoxide dismutase (SOD) and increased glutathione peroxidase (GSH-Px) activities of red cells in aged mice. In contrast, Epimedium obviously reduced the serum content and liver lip peroxide (LPO) in aged rats, and the content of lipofuscin (LF) in the cardiac muscle of aged mice. The results suggested that EF can be a potential slow-aging ingredient to increase SOD and GSH-Px activities and inhibit the formation of LPO and LF [28]. Zhang [29] found that ICA was one of the main constituents contributing to the antioxidant activity of Epimedium. Liu [30] confirmed that ICA was an antioxidant with much higher effectiveness in kinetics.
2.2.2. Against d-galactose-induced aging
Subcutaneous injection of d-galactose (D-gal) can cause the rapid aging of experimental animals, which is similar to the natural aging process [31]. To investigate the protective effects of Epimedium on D-gal-induced H9c2 cell senescence, Li [32]used D-gal-induced (50 mmol/L) to induce the aging of H9c2 cells. He found that Epimedium decreased the quantity of β-galactosidase-positive cells, the content of malonaldehyde (MDA), and the fluorescence intensity of ROS, increased the activity of SOD, and enhanced the condition of chromatin. ICA is a significant constituent of flavonoids from Epimedium, and tyrosine kinase TrkB (tropomyosin receptor kinase B) was investigated in D-gal-treated rats. By the Morris water maze, it's found that subcutaneous injection of D-gal (500 mg/kg/d) for four months can cause memory loss. Morphological abnormalities of neurons in the hippocampal region and reduced expression of brain-derived neurotrophins factor (BDNF) and TrkB were observed. D-gal-induced rat behavioral dysfunction and neurodegeneration was markedly attenuated after ICA (60 mg/kg/d) was given orally 1 h after subcutaneous injection of D-gal daily for 4 months, which was evidenced by shortened escape latency, reduced search distance and rescued morphologic abnormalities. These results clearly demonstrated that D-gal produced learning and memory deficits after chronic administration. ICA can protect neurons from D-gal insults and improve memory loss [33]in D-gal aging mice [29,34].
2.3. The regulation of metabolism
2.3.1. Cellular metabolism
Cheong [34] found that EF could stimulate the proliferation and migration of adrenocortical stem cells in corticosterone-treated rats, which was recognized as a model of Shen-yang deficiency. The study also indicated that EF could improve growth hormone (GH), insulin-like growth factor-binding protein (IGFBP), and growth hormone-releasing hormone (GHRH) in model rats through a gene chip test, which indicated that EF could remarkably upregulate the secretion of the endocrine hormone. Gao [35] demonstrated that EF could resist D-gal-induced senescence in H9c2 cells by increasing antioxidant activity and reducing apoptosis. Homocysteine significantly increased cellular senescence both in vitro and in vivo. Xiao [36] indicated that ICA delayed homocysteine-induced endothelial senescence in vitro and in vivo. Activation of the PI3K/Akt-eNOS-dependent signaling pathway may be responsible for the efficacy of ICA. In summary, all of the abovementioned results indicated that EF and its extracts could counteract the suppression of glucocorticoids on the hypothalamus-pituitary-adrenal (HPA) axis and postpone aging on stem cells, which are probably the cytological foundation. Furthermore, TCM could treat age-related diseases by activating endogenous stem cells by mobilizing and elevating hormone and cytokine levels and promoting potential organisms into energetic states. It is a novel angle for the therapy of degenerative diseases to activate endogenous stem cells in regeneration response.
Osteoporosis is characterized by low bone mineral density and an increased incidence of skeletal fractures in the aged population, especially postmenopausal women [37,38]. Medicinal herbs have been commonly used as alternative therapies in the prevention and treatment of osteoporosis under the TCM theory in China, while Epimedium is one of the most frequently used herbs [39]. According to the theory of TCM, Epimedium is a kind of herb with the function of improving the skeletal system by enhancing kidney function. Prior studies have also indicated that Epimedium could enhance bone metabolism, improve bone mineral content, and restore trabecular bone mass, architecture and bone biomechanical properties [40,41].
We searched PubMed using the following keywords: osteoporosis, postmenopausal osteoporosis, Epimedium barrenwort, Bishop's hat, fairy wings, horny goat weed, and Yin Yang Huo, and found six preclinical studies investigating the role of Epimedium in improving osteoporosis. A study found that ICA, which is a prenylated flavonol glycoside originating from Epimedium, increased the peak bone mass attained by young rats and promoted the maturation and mineralization of rat calvarial osteoblasts [42]. Another study showed that icaritin could stimulate osteogenic differentiation and inhibit adipogenesis of marrow mesenchymal stem cells [43]. Overall, preclinical studies revealed the potential effects of Epimedium in preserving bone mineral density (BMD) and improving the bone formation rate.
2.3.2. Lipid metabolism
Metabonomic understanding of Epimedium or its active ingredients can be verified through various pathways. For example, the TOR, AMPK, SIR-2 and IIS pathways and the TOR, AMPK, and SIR-2 pathways are thought to be closely related to dietary restriction. Epimedium reduced the levels of total cholesterol (TC) and triglyceride (TG) in hyperlipidemic rats. Hu [44] divided 70 rats into five groups and gave them daily intragastric administration of normal saline, simvastatin, or ICA (30 mg/kg/d, 60 mg/kg/d) for 4 weeks. The levels of blood lipids, SOD, and MDA were measured. The results indicated that the levels of blood lipids, including TC, TG, low-density lipoprotein-cholesterol (LDL-C), and MDA, were significantly increased. In contrast, high-density lipoprotein-cholesterol (HDL-C) and SOD were significantly decreased. The mechanisms may be associated with the anti-inflammatory and antioxidative stress effects of ICA. It has been reported to be effective for treating a variety of cardiovascular diseases by lowering blood fat. Liquid chromatography coupled with mass spectrometry (LC/MS) based on a metabolomics approach was applied to characterize the aging of rats by Yan [45]. Serum samples from the 4 groups were collected at 4, 10, 18 and 24 months, and rats treated with TFE were profiled by LC/MS. Critical age-related metabolites, such as unsaturated fatty acids, saturated fatty acids, and amino acids, displayed age-related changes. Most of them were reset to a younger level with administered TFE. This investigation indicates that aging is identified by a series of diversifications in lipid metabolism and free radical accumulation, and TFE is expected to be a promising novel slow-aging ingredient, considering that its slow-aging effects might be the result of the regulation of lipid metabolism and antioxidation properties.
To investigate the effects of aging on rat urinary metabolites and to evaluate the slow-aging effects of Epimedium, Wu [46] chose 4-, 10-, 18- and 24-month-old rats that were administered TFE. Based on current metabonomic analyses, the results indicated that 26 characteristic resonances were highly related to aging. Additionally, distinct resonances were reset to mainly younger groups after intervention by TFE. These metabolites involved creatinine metabolites, aliphatic amine metabolites and some important intermediates or end products of energy metabolism. This result indicates that intervention by TFE can postpone the process of aging and shows antiaging effects, which might be due to the amelioration of pyruvate metabolism and oxidative phosphorylation. According to the above studies, Epimedium is considered to be a promising potential slow-aging medicine.
2.3.3. Bone metabolism
We identified four clinical trials examining the role of Epimediumin bettering osteoporosis in English by searching PubMed. A 24-month randomized, double-blind placebo-controlled clinical trial evaluated the effects of Epimedium-derived phytoestrogen flavonoids (EPFs) on BMD in postmenopausal women in Hong Kong, China [8]. One hundred healthy late postmenopausal women were randomized into the EPF treatment group (n = 50; a daily dose of 60 mg ICA, 15 mg daidzein, and 3 mg genistein) or placebo control group (n = 50). EPFs maintained BMD at 12 months and 24 months, while BMD decreased in the control group, with statistical significance between the groups [8]. However, either serum estradiol or endometrial thickness was found to be changed in each group [8]. Another clinical study exploring the clinical efficacy of medicinal cake-separated moxibustion for senile osteoporosis [39] found that Epimedium, as a medicine for external application, which is the main component of the medical cake, was able to improve the symptoms of senile osteoporosis patients, enhance BMD, and lower serum β-type I collagen carboxy-terminal peptide. Another 5-year multicenter follow-up study enrolled 194 postmenopausal women in Mainland China, comparing kidney-tonifying herbal Fufang with phytoestrogenic Epimedium (10 g/day, twice per day, n = 101) and placebo (n = 93) [47]. All patients were supplemented daily with calcium and vitamin [47]. BMD increased significantly from baseline in the kidney-tonifying herbal Fufang group at 5 years, while BMD decreased in the placebo group (p < 0.05); the fracture incidence was lower in the kidney-tonifying herbal Fufang group, with a relative risk of 0.57 (95 % confidential interval, 0.43–0.70, p < 0.05) [47].
2.4. The modulation of the immune system
2.4.1. T lymphocytes and B lymphocytes
Cellular immune system aging, remarkably immune senescence, and T cell aging initiated by thymic involution sources of chronic inflammation in the elderly (termed inflammation), potentially induces an acceleration of brain aging and memory loss [48]. The overall change in the function of the immune system, which increases with age, is called immune senescence. Immune senescence results from multiple factors in the internal and external environment, in which T lymphocytes and B lymphocytes play a crucial role in the development of immune senescence [17,49,50]. Furthermore, Rhew [51] indicated that ICA had an immunoadjuvant effect that may enhance the immune response as an adjuvant. A study showed that treatment of ICA at 10 mg/kg/day in mice could suppress the immune response with prolonged allograft skin survival [52]. In patients who needed maintenance hemodialysis, Wang [53] found that Epimedium could significantly increase the CD3+, CD4+, CD8+, CD19+ and NK cells counts in spleen (P < 0.05) and increasing bone marrow cells. In the delayed-type hypersensitivity mouse model, Epimedium increased the CD4+ level of T-lymphocyte subpopulations. In addition, Epimedium can rebuild the immune homeostasis of T lymphocyte apoptosis and retard immune senescence, the mechanism of which may be that it can suppress the excessive apoptosis of splenic lymphocytes in aged rats and activate Rel/NF-kappaB/I B/IKK and their signal transduction pathway to upregulate NF-kappaB through adjusting I Bepsilon and I Balpha.
2.4.2. Immuno-homeostasis
The vital gene background of immune homeostasis imbalance is connected with the expression pattern and downregulation of apoptosis, inhibiting gene expression in aged individuals. Epimedium can reverse abnormal changes in gene expression with opposite functions, such as promoting and inhibiting apoptosis and enhancing and antagonizing proliferation genes, to remodel immune homeostasis in aged individuals [21].
2.4.3. Neuroendocrine-immune network
In Cai's study [54], they tested the slow-aging properties of ICA and its three derivatives, icariside I, icariside II and icaritin, in C. elegans. They found that ICA and one of its derivatives, icariside II, prolonged adult lifespan. Additionally, they discovered that icariside II delayed age-associated phenotypes in a rodent study, suggesting that icariside II significantly enhanced healthy aging. They indicated that Icariside II might act through the IIS pathway to affect the lifespan of C. elegans. To investigate the effects of ICA on the mRNA expression of BDNF and TrkB in the hippocampus of natural aging rats. BDNF belongs to the neurotrophin family, which acts on neuronal survival and growth and has been associated with the cognitive process [33]. The study suggested that the stress paradigm and aging certainly had effects on the regulation of BDNF mRNA and TrkB mRNA, which might be related to damage and protective function of the hippocampus. ICA treatment improved the arrangement of neurons and attenuated neuronal degeneration. Compared with the control group, ICA increased the expression of BDNF and TrkB mRNA in the hippocampus of aging rats. The antiaging effects of ICA might be related to the increased BDNF and TrkB mRNA expression in the hippocampus. Zhang [55] found that ICA can increase mitochondrial activity, inhibit beta-amyloid (Abeta) production, and enhance the expression of neurotrophic factors in the brains of model rats induced by sodium azide. Epimedium may prolong adult lifespan through the IIS pathway, increase the expression of BDNF and TrkB mRNA in the hippocampus during aging and increase the expression of neurotrophic factors in the brain.
2.4.4. Inflammation-aging
The natural aging process is associated with chronic, low-grade, mild inflammation. This phenomenon is called inflammaging (inflammation-aging) [35,36,44]. Epimedium has a wide range of anti-inflammatory, antibacterial and antiviral activities. The results of Ti's experiment revealed that compound 6 was the most potent of the 10 different compounds tested in the current study, with a maximal inhibitory ratio of 79 % in vitro anti-inflammatory activity [45]. Antibacterial experiments have shown that diphylloside A, icarisoside A, and desmethylanhydroicaritin have significant activity toward bacteria [46]. Epimedium has definite anti-inflammatory, antibacterial and antiviral effects and has many functions. Inflammation-aging is now thought to be related to chronic diseases, such as Parkinson's and Alzheimer's disease (AD), atherosclerosis, and type 2 diabetes, and Epimedium may have certain prospects in preventing these diseases.
2.5. The regulation of sex hormone
Sex hormones are closely related to aging. In men, steroid hormone levels are positively correlated with aging [56], so the rate of steroid hormone change can be used as a biomarker for healthy aging [57]. In women, declining ovarian function across the stages of the menopause transition contributes to vascular aging [58]. Estrogens were reported to be neuroprotective, and a decrease in their level may be related to many neurodegenerative diseases [59]. The level of sex hormones directly affects sexual dysfunction; however, sexual dysfunctions and aging are inexorably related in both men and women, but aging-related sexual dysfunctions are poorly investigated [[60], [61], [62]]. Epimedium means high libido in Chinese and has been used as a male enhancement supplement and infertility therapy for a long history under TCM theory. According to TCM, renal dysfunction is the leading cause of sexual dysfunction. Epimedium can enhance renal functions to improve sexual functions and can be combined with Western medicine in clinical practice.
Age-related changes in psychological, physical, and sexual functions in men are partially explained by testosterone deficiency [63,64]. Most clinical symptoms in aged men can be categorized as sexual or nonsexual [64]. Nonsexual symptoms include decreased energy or motivation, feeling sad or blue, poor concentration and memory, reduced muscle bulk, increased body fat, and diminished work performance [65,66]. Sexual symptoms include erectile dysfunction (ED) and decreased libido, frequency of sexual desire, and nocturnal erections [48,67]. ED, the inability to reach or maintain an erection to satisfy sexual intercourse sufficiently, is common among aged men [68]. In addition to increasing age, major causes of ED also include cigarette smoking, obesity, diabetes mellitus, hypertension, and depression [69,70]. Standard, efficient, and safe therapies for ED are still hardly reached [69,71,72]. Alternative treatments for ED, including acupuncture and herbs, are becoming popular worldwide. Epimedium may serve as a potential inhibitor of phosphodiesterase type 5 (PDE5) [73,74].
2.5.1. Males
We found two clinical trials investigating the effects of Epimedium on symptom improvement in aged males by searching PubMed. A randomized, double-blind, placebo-controlled crossover study of herbal medicine including Epimedium was conducted for the treatment of ED in Thailand in 2013 [75]. Sixty-one adult patients with mild to moderate ED were randomized to receive herbal medicine with Epimedium or identical-looking placebo: patients were randomized to receive herbal medicine for two weeks and were switched to receive placebo for another two weeks, with a one-week washout period between the two periods, and vice versa for the other group [75]. A questionnaire named IIEF (The International Index of Erectile Function) was used to assess the effects of therapy. IIEF is a questionnaire which used to evaluate the ED, such as orgasmic function, erectile function, intercourse satisfaction, sexual desire, and overall satisfaction [75]. At baseline and the end of each treatment IIEF scores were collected. Herbal medicine showed a higher trend of improvement in the IIEF score for all domains compared with placebo (only with a statistically significant difference in the field of erectile function) [75]. Another open-label, randomized clinical trial compared the effects of a preparation containingEpimedium and Kampo (approved for reimbursement in Japan but different than Epimedium in TCM) in Japan [76]. Forty-nine aging male patients with mild or severe symptoms were enrolled and randomly assigned to the Epimedium or Kampo groups for 6 months. Patients receiving Epimedium showed better somatic and psychological signs of aging, including ED, than their baseline and the Kampo group [76].
2.5.2. Females
Approximately 1.5 million women experience menopause transition symptoms each year, and major symptoms of menopause include vasomotor symptoms, vulvovaginal atrophy, insomnia and adverse mood [77,78]. Clinical trials indicate the efficiency of estrogens in reducing vasomotor and sexual symptoms but increasing the risk of adverse effects of having breast cancer, stroke, venous thromboembolism, and endometrial cancer [77,79]. Epimedium is also widely used in Asia as alternative medicine in menopausal women to improve menopausal transition symptoms by enhancing renal and hepatic functions according to TCM's theory.
We found only one clinical study investigating the effects of Epimedium in menopause by searching PubMed [80]. Ninety women were randomized into a 6-month Epimedium water extract therapy group and a 6-month water placebo control group [80]. The results indicated that the components of Epimediumcould significantly increase the level of serum estradiol (p < 0.01) [80].
3. Efficacy
We summarized some clinical trials of Epimedium to observe its clinical efficacy. Still, these clinical trials have a small sample size and lack large-scale randomized controlled clinical trials andmulticenter trials.
3.1. Sexual function
Epimedium has a good effect on improving sexual function. In China, Zhuang collected 20 cases from middle-aged ED patients and divided them into treatment and control groups. The treatment group used the anhydroicaritin (AHI) homolog (extracted from Epimedium),and the results showed that AHI has therapeutic efficacy in middle-aged men in the ED (P > 0.05) [81]. Sixty-one adult patients with mild to moderate ED were randomized to receive herbal medicine with Epimedium or identical-looking placebo: patients were randomized to receive herbal medicine for two weeks and were switched to receive placebo for another two weeks, with a one-week washout period between the two periods and vice versa for the other group [75]. Use the IIEF to evaluate the improvement of ED. IIEF scores were collected at baseline and the end of each treatment, and herbal medicine showed a higher trend of improvement in the IIEF score for all domains compared with placebo [75]. Another six-month study (including 49 aging males) in Japan showed that Epimedium has better somatic and psychological symptoms of aging, including ED, than their baseline and the Kampo group [76]. In addition to oral administration, external application of Epimedium compound (containing 3 % peppermint oil and 30 % ICA) can play a role in local anesthesia, reduce the sensitivity of the penis head, and have a good therapeutic effect on premature ejaculation [82].
3.2. Osteoporosis
Epimedium also has a good curative effect in treating osteoporosis and delaying postmenopausal bone loss. A 24-month randomized double-blind placebo-controlled clinical trial (one hundred women were included) evaluating the effects of EPFs on BMD in postmenopausal women was carried out in Hong Kong, China [8]. Another 5-year multicenter follow-up study enrolled 194 postmenopausal women in mainland China, comparing kidney-tonifying herbal Fufang with phytoestrogenic Epimedium (n = 101) and placebo (n = 93) [47]. BMD increased significantly from baseline in the kidney-tonifying herbal Fufang group at 5 years, while BMD decreased in the placebo group (p < 0.05); the fracture incidence was lower in the kidney-tonifying herbal Fufang group, with a relative risk of 0.57 (p < 0.05) [47]. EPFs maintained BMD at 12 months and 24 months, while BMD decreased in the control group, with statistical significance between the groups. However, either serum estradiol or endometrial thickness was found to change in each group. Epimedium, as a medicine for external application, which is the main component of the medical care, was able to improve the symptoms of senile osteoporosis patients, enhance BMD, and lower the serum β-type I collagen carboxy-terminal peptide [39].
3.3. Coronary heart disease
Atherosclerosis is the common pathological basis of ischemic cardiovascular and cerebrovascular diseases. One hundred twenty-aged patients with kidney deficiency syndrome of ischemic cardiocerebral vascular diseases (60 cases of coronary heart disease and 60 cases of cerebral atherosclerosis) were treated with Epimedium compound granules [83]. The results showed that after the therapeutic period, the total and marked effective rates were 96.7 % and 39.5 %, respectively, in the treatment group. The improvement rates were 70 % in electrocardiograms of patients with coronary heart diseases and 75 % in electroencephalograms of patients with cerebral arteriosclerosis.Epimedium compound granules can also significantly reduce the content of serum cholesterol and TC, and increase the content of HDL-C. It can obviously increase the activity of serum SOD and reduce the content of MDA. The therapeutic effectiveness in the treatment group was better than that in the control group treated with Su-Guan-Bian. It proves that Epimedium compound granules has the function of preventing and treating atherosclerosis. According to experimental observations, the therapeutic effectiveness was related to the fact that Epimedium compound granules with lower blood lipids have anti-free radicals and adjust the balance between prostacyctin I2 and thromboxane A2 (TXA2/PGI2).
4. Adverse reactions
Although Epimedium is listed as both a food and a medicine, it is not definitely safe. In recent years, many studies have reported the adverse effects of Epimedium and its related preparations, but no significant adverse effects have been discovered. A study evaluated the safety range of the water extract of Epimedium and found that its LD50 was above 80 g/kg; its IC50 values in Chinese hamster ovary cells and lung cells were 55.4 and 19.53 mg/ml, respectively. In addition, the study also found that all toxicity tests were negative [84]. The long-term toxicity of the TFE was also measured. Using a typical Wistar rat model, a study discovered that the dosage of long-term toxicity was 410 g/kg/day for 12 weeks, and no significant pathology changes were observed [85]. The genetic toxicity of the water extract of Epimedium was tested and showed that the herb is nongenotoxic [86]. In terms of adverse drug reactions, an animal test showed that after 3 days of administration of Epimedium in mice, symptoms of vomiting, poor appetite, and decreased activities occurred [84]. After being administered for 15 days, fatty degeneration of the liver ensued. In addition, Epimedium has androgen-like action, and androgen often has certain hepatotoxicity; therefore, it can be inferred that Epimedium may have certain hepatotoxicity [87].
5. Application status of Epimedium
To the best of our knowledge, almost all clinical trials of Epimedium have been carried out in Asian countries, mainly in China. A limited number of English-written articles contributed to the lack of generality. Well-designed and well-conducted studies should be carried out to test the effects of Epimedium in clinical applications. We stated the investigational clinical status of Epimedium in this section (Table 1). Although there are no sufficient large-scale clinical trials to provide evidence of Epimedium in clinical practice, we wish to win global interest in Epimedium as a natural slow-aging herb.
Table 1.
Summary of clinical trials of Epimedium.
| Clinical Trial | Study Design | Study Subjects | Subject Number | Formulation | Dosing | Effective Outcomes | Safety Outcomes |
|---|---|---|---|---|---|---|---|
| Zhang et al., 2007 (8) | RCT | Healthy late postmenopausal women (mean age 64y) |
100 | Capsule, composing 15 mg Icariin | 4 capsules, daily | Beneficial effect on preventing bone loss | Neither dominant side effects on major systems nor abnormal hematology indicators were found |
| Deng et al., 2012 (48) | RCT | Postmenopausal Osteoporosis (47–70y) |
194 | Herbal Fufang | 10 g/day, twice daily | Beneficial effect on preventing bone loss | No notable adverse events were observed |
| Punyawudh et al., 2013 (76) | Randomized, double-blind, placebo-controlled, crossover study | Patients with mild or mild to moderate ED (≥ 18y) | 63 | Tablet, composing Epimedium Drevicornum Maxim 120 g | 1 tablet 1h before planned sexual activity | Improvements in mild to moderate ED | Dizziness (13.3 %), face numbness (1.6 %), and tachycardia (1.6 %) |
| Nishimatsu et al., 2014 (77) | RCT | Male patients with mild or moderate symptoms of aging (mean age 63y) | 94 | Capsule, composing 5 mg of Epimedium herb extract | 1 capsule, twice daily | Improvement in symptoms of aging, including ED, in males | Epigastric discomfort (4 %) and skin rash (4 %) |
| Yan et al., 2008 (81) | RCT | Normal postmenopausal subjects (mean age 57y) | 90 | Herba Epimedii water extract | 300 mL, daily | Decreased the TC and TG levels; increased the serum level of E2 | No serious adverse effects were observed |
| Zhao et al., 2012 (91) | RCT | Patients with stable moderate or severe COPD (58–90y) | 90 | 30 g ShanYao and 12 g Herba Epimedii, immersed in water for 30 min, decocted twice, then filtered |
80 ml, twice daily | Improved in dyspnea, exercise capacity, and quality of life | No serious adverse effects were observed |
| Cai et al., 1998 (92) | RCT | Patients took prednisone (age not reported) | 65 | EB 5g | 5g daily | EB could relieve the neuroendocrine-immunological effect inhibited by exogenous glucocorticoid | Not reported |
| Liao et al., 1995 (93) | Controlled trial | Patients of hemodialysis maintenance (age not reported) | 34 | ES | 0.6 g/Kg daily | Therapeutic effect on the sexual disorder and immunologic inadequacy | Not reported |
RCT: randomized controlled trial; ED: Erectile dysfunction; TC: cholesterol; TG: triglyceride; E2: serum estradiol; EB: Epimedium brevicornum; ES: Epimedium Sagittatum; COPD: chronic obstructive pulmonary disease.
6. Conclusions
Aging-related diseases have become a significant public health problem globally, and considerable progress has been achieved in the field of Western slow-aging agents. Natural plants, especially Chinese herbal medicine, have built up a characteristic medical system directed by traditional Chinese medical theory and have provided rational means for various diseases, including aging. Herbal Epimedium species have been widely used in TCM for sexual enhancement, immunity improvement, and slow-aging treatment, with flavonoids and polysaccharides being the major active components. In this review, we summarized thepotential mechanisms of its slow-aging activity and randomized controlled trials on the effectiveness and safety of Epimedium. Some studies have demonstrated the slow-aging effects of EF and ICA on aging. We also showed the adverse effects of Epimedium in clinical trials and analyzed the reasons.In summary, the dosage of Epimedium ranges from 250 to 500 mg, but the ideal dosage remains unknown. There are no major contraindications when using this herb, but clinical trials to test long-term adverse reactions are lacking. Epimedium is an herbal medicine to stimulate kidney Yang in the TCM theory, and its hormone-like action may cause some potential side effects that need further research. Due to the developments of modern pharmacological investigations on EF and ICA. Currently, Epimedium is widely used in the clinic for aging-related diseases in the real world in China; however, large-scale studies are still needed; therefore, we need to study more about it and use it more appropriately.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contribution statement
ZW and SN contributed equally to this work. SJ and ZW designed the search strategy; GC and LS conducted the search of the studies; ZY and WH screened the studies; TX evaluated the studies; SN and ZW wrote the article; SJ was mainly responsible for the final check of the article. All authors read and approved the final version of the manuscript.
Funding statement
This work was supported by the Key Special Project of Ministry of Science and Technology Research on modernization of Traditional Chinese Medicine (2019YFC1712400) and Beijing Traditional Chinese Medicine Science and Technology Development Fund (JJ-2020-21)and Beijing Traditional Chinese Medicine Science and Technology Development Fund Project (BJZYYB-2023-47).
Declaration of competing interest
All authors have read and approved the manuscript. The authors have no relevant conflicts of interests. The manuscript has not been submitted elsewhere nor published elsewhere in whole or in part. It is not under consideration for publication elsewhere, that its publication is approved by all authors. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder. All authors have approved the final article.
Contributor Information
Xiaolin Tong, Email: Tongxiaolin@vip.163.com.
Juexian Song, Email: songjuexian@vip.163.com.
References
- 1.Seals D.R., Justice J.N., LaRocca T.J. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui T., Kovell R.C., Brooks D.C., Terlecki R.P. A urologist's guide to ingredients found in top-selling nutraceuticals for men's sexual health. J. Sex. Med. 2015;12:2105–2117. doi: 10.1111/jsm.13013. [DOI] [PubMed] [Google Scholar]
- 3.Zhai M., He L., Ju X., Shao L., Li G., Zhang Y., Liu Y., Zhao H. Icariin acts as a potential agent for preventing cardiac ischemia/reperfusion injury. Cell Biochem. Biophys. 2015;72:589–597. doi: 10.1007/s12013-014-0506-3. [DOI] [PubMed] [Google Scholar]
- 4.Colman R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W., Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y., Li Y., Liu C., Li E., Gao Z., Liu C., Gu W., Huang Y., Liu J., Wang D., Hu Y. Structural characterization of an acidic Epimedium polysaccharide and its immune-enhancement activity. Carbohydr. Polym. 2016;138:134–142. doi: 10.1016/j.carbpol.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Xue L., Jiang Y., Han T., Zhang N., Qin L., Xin H., Zhang Q. Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu maxim. J. Ethnopharmacol. 2016;192:370–381. doi: 10.1016/j.jep.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Meng F.H., Li Y.B., Xiong Z.L., Jiang Z.M., Li F.M. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine. 2005;12:189–193. doi: 10.1016/j.phymed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G., Qin L., Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J. Bone Miner. Res. 2007;22:1072–1079. doi: 10.1359/jbmr.070405. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C.Z., Wang S.X., Zhang Y., Chen J.P., Liang X.M. In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. J. Ethnopharmacol. 2005;98:295–300. doi: 10.1016/j.jep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Li J., Wang Y., Liang Q. Taxonomy of Epimedium (Berberidaceae) with special reference to Chinese species. Chin Herb Med. 2022;14:20–35. doi: 10.1016/j.chmed.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei L.K., Guo B.L., Sun S.Q., Huang W.H. [Study on the identification of some species of Herba Epimedii with FTIR] Guang Pu Xue Yu Guang Pu Fen Xi. 2008;28:55–60. [PubMed] [Google Scholar]
- 12.Jiang J., Li J., Zhang Z., Sun E., Feng L., Jia X. Mechanism of enhanced antiosteoporosis effect of circinal-icaritin by self-assembled nanomicelles in vivo with suet oil and sodium deoxycholate. Int J Nanomedicine. 2015;10:2377–2389. doi: 10.2147/IJN.S76191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei J.J., Zhang J.K., Li M., Xie S.S., Tao S.Q., Yang Y. A new flavonoid glycoside from Epimedium sagittatum. Acta Pharm. Sin. 2023;58:180–185. [Google Scholar]
- 14.Wang L., Li Y., Guo Y., Ma R., Fu M., Niu J., Gao S., Zhang D. Herba epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr Pharm Des. 2016;22:328–349. doi: 10.2174/1381612822666151112145907. [DOI] [PubMed] [Google Scholar]
- 15.Ma H., He X., Yang Y., Li M., Hao D., Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011;134:519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D.W., Cheng Y., Wang N.L., Zhang J.C., Yang M.S., Yao X.S. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine. 2008;15:55–61. doi: 10.1016/j.phymed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Huang J.H., Shen Z.Y., Wu B. Effect and mechanism of Epimedium flavanoids for aging retardation from viewpoint of transcriptomics and metabonomics. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:47–50. [PubMed] [Google Scholar]
- 18.Hu Z.W., Shen Z.Y., Huang J.H. Experimental study on effect of epimedium flavonoids in protecting telomere length of senescence cells HU. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:1094–1097. [PubMed] [Google Scholar]
- 19.Arantes-Oliveira N., Apfeld J., Dillin A., Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 20.Liu X.Y., Wang Q., Xia S.J., Huang J.H., Shen Z.Y., Xu H. Characteristics of lymphocyte nuclear factor-kappaB signal transduction kinase expression in aging process and regulatory effect of epimedium flavonoids. Chin. J. Integr. Med. 2011;17:704–709. doi: 10.1007/s11655-011-0848-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Shen Z.Y., Chen W.H. Molecular mechanism of epimedium flavonoids in immune homeostasis remodeling in aged rats revealed by lymphocyte gene expression profile. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:59–62. [PubMed] [Google Scholar]
- 22.Sze S.C., Tong Y., Ng T.B., Cheng C.L., Cheung H.P. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010;15:7861–7870. doi: 10.3390/molecules15117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X., Zhao H., Yang S., Ma Y., Zhang Y., Yang Y. Icariin attenuates DNA damage in testicular germ cells of natural aging rats by activating Nrf2/HO-1 signaling pathway. Chin. Tradit. Herb. Drugs. 2019;50:7. [Google Scholar]
- 24.Zhao H., Song L., Huang W., Liu J., Yuan D., Wang Y., Zhang C. vol. 49. Andrologia; 2017. Total Flavonoids of Epimedium Reduce Ageing-Related Oxidative DNA Damage in Testis of Rats via P53-dependent Pathway. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J.L., Ding H., Song X.B. Research progress on anti-aging effect of total flavonoids of herba epimedii. Nat Prod Res Dev. 2018;30:339–343+309. [Google Scholar]
- 26.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 27.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y., Hou Y., Tang G., Cai E., Liu S., Yang H., Zhang L., Wang S. Optimization of ultrasonic extraction of phenolic compounds from epimedium brevicornum maxim using response surface methodology and evaluation of its antioxidant activities in vitro. J Anal Methods Chem. 2014;2014 doi: 10.1155/2014/864654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Chen H., Wang Z., Lan G., Zhang L. Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. J. Food Sci. Technol. 2013;50:1122–1129. doi: 10.1007/s13197-011-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z.Q. Icariin: a special antioxidant to protect linoleic acid against free-radical-induced peroxidation in micelles. J. Phys. Chem. A. 2006;110:6372–6378. doi: 10.1021/jp053998z. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto A.M., Marck B.T., Gruenewald D.A., Wolden-Hanson T., Naai M.A. Aging and the neuroendocrine regulation of reproduction and body weight. Exp. Gerontol. 2000;35:1251–1265. doi: 10.1016/s0531-5565(00)00158-3. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Deng L.L., Zhou Z.Y., Yuan D., Zhang C.C., Wang T. Protective effect of total saponins of Panax notoginseng combined with total flavonoids of epimedium on D-galactose-incuced senescence of H9c2 cell. Zhongguo Zhongyao Zazhi. 2017;42:555–561. doi: 10.19540/j.cnki.cjcmm.2017.0012. [DOI] [PubMed] [Google Scholar]
- 33.Shao S.H., Shi S.S., Li Z.L., Zhao M.S., Xie S.Y., Pan F. Aging effects on the BDNF mRNA and TrkB mRNA expression of the hippocampus in different durations of stress. Chin. J. Physiol. 2010;53:285–293. doi: 10.4077/cjp.2010.amk056. [DOI] [PubMed] [Google Scholar]
- 34.Cheong L.Z., Sun T., Li Y., Zhou J., Lu C., Li Y., Huang Z., Su X. Dietary krill oil enhances neurocognitive functions and modulates proteomic changes in brain tissues of d-galactose induced aging mice. Food Funct. 2017;8:2038–2045. doi: 10.1039/c6fo01848c. [DOI] [PubMed] [Google Scholar]
- 35.Gao S.M., Wang L., Shi Y.X., Ju C.X., Zhang F., Li F.X. Protective effects of total epimedium flavonoids against QA-induced toxicity in SH-SY5Y cells. Zhong Yao Cai. 2013;36:1978–1982. [PubMed] [Google Scholar]
- 36.Xiao-Hong D., Chang-Qin X., Jian-Hua H., Wen-Jiang Z., Bing S. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharm. Biol. 2013;51:433–440. doi: 10.3109/13880209.2012.738332. [DOI] [PubMed] [Google Scholar]
- 37.Black D.M., Rosen C.J. Clinical practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 38.Gosch M., Kammerlander C., Nicholas J.A. Treatment of osteoporosis in older adults. Panminerva Med. 2014;56:133–143. [PubMed] [Google Scholar]
- 39.Ma R., Zhu R., Wang L., Guo Y., Liu C., Liu H., Liu F., Li H., Li Y., Fu M., Zhang D. Diabetic osteoporosis: a review of its traditional Chinese medicinal use and clinical and preclinical research. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/3218313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y.Q., Han X.F., Liu T., Cheng M.C., Xiao H.B. A cell-based model of bone remodeling for identifying activity of icarrin in the treatment of osteoporosis. Biotechnol. Lett. 2015;37:219–226. doi: 10.1007/s10529-014-1661-8. [DOI] [PubMed] [Google Scholar]
- 41.Peng S., Zhang G., Zhang B.T., Guo B., He Y., Bakker A.J., Pan X., Zhen W., Hung L., Qin L., Leung W.N. The beneficial effect of icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone. 2013;55:230–240. doi: 10.1016/j.bone.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Shi W., Gao Y., Wang Y., Zhou J., Wei Z., Ma X., Ma H., Xian C.J., Wang J., Chen K. The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts. J. Biol. Chem. 2017;292:20883–20896. doi: 10.1074/jbc.M117.809517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng H., Rui X.F., Sheng C.J., Li W.J., Cheng X.Y., Jhummon N.P., Yu Y.C., Qu S., Zhang G., Qin L. A novel semisynthetic molecule icaritin stimulates osteogenic differentiation and inhibits adipogenesis of mesenchymal stem cells. Int. J. Med. Sci. 2013;10:782–789. doi: 10.7150/ijms.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y., Sun B., Liu K., Yan M., Zhang Y., Miao C., Ren L. Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway. Inflammation. 2016;39:228–236. doi: 10.1007/s10753-015-0242-x. [DOI] [PubMed] [Google Scholar]
- 45.Yan S., Wu B., Lin Z., Jin H., Huang J., Yang Y., Zhang X., Shen Z., Zhang W. Metabonomic characterization of aging and investigation on the anti-aging effects of total flavones of Epimedium. Mol. Biosyst. 2009;5:1204–1213. doi: 10.1039/b816407j. [DOI] [PubMed] [Google Scholar]
- 46.Wu B., Yan S., Lin Z., Wang Q., Yang Y., Yang G., Shen Z., Zhang W. Metabonomic study on ageing: NMR-based investigation into rat urinary metabolites and the effect of the total flavone of Epimedium. Mol. Biosyst. 2008;4:855–861. doi: 10.1039/b800923f. [DOI] [PubMed] [Google Scholar]
- 47.Deng W.M., Zhang P., Huang H., Shen Y.G., Yang Q.H., Cui W.L., He Y.S., Wei S., Ye Z., Liu F., Qin L. Five-year follow-up study of a kidney-tonifying herbal Fufang for prevention of postmenopausal osteoporosis and fragility fractures. J Bone Miner Metab. 2012;30:517–524. doi: 10.1007/s00774-012-0351-7. [DOI] [PubMed] [Google Scholar]
- 48.Albersen M., Orabi H., Lue T.F. Evaluation and treatment of erectile dysfunction in the aging male: a mini-review. Gerontology. 2012;58:3–14. doi: 10.1159/000329598. [DOI] [PubMed] [Google Scholar]
- 49.Morkve O., Laerum O.D. Flow cytometric measurement of p53 protein expression and DNA content in paraffin-embedded tissue from bronchial carcinomas. Cytometry. 1991;12:438–444. doi: 10.1002/cyto.990120509. [DOI] [PubMed] [Google Scholar]
- 50.Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 51.Rhew K.Y., Han Y. Immunoadjuvant activity of icariin that induces Th1-type antibody in mice. Arch Pharm. Res. (Seoul) 2012;35:1685–1691. doi: 10.1007/s12272-012-0920-2. [DOI] [PubMed] [Google Scholar]
- 52.Li X., Hu Y., He L., Wang S., Zhou H., Liu S. Icaritin inhibits T cell activation and prolongs skin allograft survival in mice. Int Immunopharmacol. 2012;13:1–7. doi: 10.1016/j.intimp.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Wang H.W., Jia L.L., Xu Y.Q., Zeng X., He Y.M., Yuan D. Immunoregulatory effects of total flavones of epimedium on immunosuppression mice. Tianjin Med. J. 2010;38:1068–1071. [Google Scholar]
- 54.Cai J., Zheng T., Zhang L., Tian Y., Yang M.H., Du J. Effects of Herba epimedii and Fructus ligustri lucidi on the transcription factors in hypothalamus of aged rats. Chin. J. Integr. Med. 2011;17:758–763. doi: 10.1007/s11655-011-0636-z. [DOI] [PubMed] [Google Scholar]
- 55.Li L., Li L., Xie F., Zhang Z., Guo Y., Tang G., Lv D., Lu Q., Chen L., Li J. Jagged-1/Notch3 signaling transduction pathway is involved in apelin-13-induced vascular smooth muscle cells proliferation. Acta Biochim. Biophys. Sin. 2013;45:875–881. doi: 10.1093/abbs/gmt085. [DOI] [PubMed] [Google Scholar]
- 56.Feldman H.A., Longcope C., Derby C.A., Johannes C.B., Araujo A.B., Coviello A.D., Bremner W.J., McKinlay J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 57.Walther A., Philipp M., Lozza N., Ehlert U. The rate of change in declining steroid hormones: a new parameter of healthy aging in men? Oncotarget. 2016;7:60844–60857. doi: 10.18632/oncotarget.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau K.L. Modulatory influence of sex hormones on vascular aging. Am. J. Physiol. Heart Circ. Physiol. 2019;316:522–526. doi: 10.1152/ajpheart.00745.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vegeto E., Villa A., Della Torre S., Crippa V., Rusmini P., Cristofani R., Galbiati M., Maggi A., Poletti A. The role of sex and sex hormones in neurodegenerative diseases. Endocr. Rev. 2020:41. doi: 10.1210/endrev/bnz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minkin M.J. Sexual health and relationships after age 60. Maturitas. 2016;83:27–32. doi: 10.1016/j.maturitas.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Clayton A.H., Harsh V. Sexual function across aging. Curr Psychiatry Rep. 2016;18:28. doi: 10.1007/s11920-016-0661-x. [DOI] [PubMed] [Google Scholar]
- 62.Ni Lochlainn M., Kenny R.A. Sexual activity and aging. J. Am. Med. Dir. Assoc. 2013;14:565–572. doi: 10.1016/j.jamda.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 63.Nigro N., Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med. Wkly. 2012;142 doi: 10.4414/smw.2012.13539. [DOI] [PubMed] [Google Scholar]
- 64.McBride J.A., Carson C.C., 3rd, Coward R.M. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8:47–60. doi: 10.1177/1756287215612961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buvat J., Maggi M., Gooren L., Guay A.T., Kaufman J., Morgentaler A., Schulman C., Tan H.M., Torres L.O., Yassin A., Zitzmann M. Endocrine aspects of male sexual dysfunctions. J. Sex. Med. 2010;7:1627–1656. doi: 10.1111/j.1743-6109.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 66.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S., Montori V.M., Task Force E.S. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 67.Morelli A., Corona G., Filippi S., Ambrosini S., Forti G., Vignozzi L., Maggi M. Which patients with sexual dysfunction are suitable for testosterone replacement therapy? J. Endocrinol. Invest. 2007;30:880–888. doi: 10.1007/BF03349232. [DOI] [PubMed] [Google Scholar]
- 68.Montorsi F., Adaikan G., Becher E., Giuliano F., Khoury S., Lue T.F., Sharlip I., Althof S.E., Andersson K.E., Brock G., Broderick G., Burnett A., Buvat J., Dean J., Donatucci C., Eardley I., Fugl-Meyer K.S., Goldstein I., Hackett G., Hatzichristou D., Hellstrom W., Incrocci L., Jackson G., Kadioglu A., Levine L., Lewis R.W., Maggi M., McCabe M., McMahon C.G., Montague D., Montorsi P., Mulhall J., Pfaus J., Porst H., Ralph D., Rosen R., Rowland D., Sadeghi-Nejad H., Shabsigh R., Stief C., Vardi Y., Wallen K., Wasserman M. Summary of the recommendations on sexual dysfunctions in men. J. Sex. Med. 2010;7:3572–3588. doi: 10.1111/j.1743-6109.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- 69.Khera M., Goldstein I. Erectile dysfunction. BMJ Clin Evid. 2011;2011 [PMC free article] [PubMed] [Google Scholar]
- 70.Guay A.T., Spark R.F., Bansal S., Cunningham G.R., Goodman N.F., Nankin H.R., Petak S.M., Perez J.B., American F. Association of Clinical Endocrinologists Male Sexual Dysfunction Task, American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: a couple's problem--2003 update. Endocr. Pract. 2003;9:77–95. doi: 10.4158/EP.9.1.77. [DOI] [PubMed] [Google Scholar]
- 71.Van Asseldonk B., Barkin J., Elterman D.S. Medical therapy for benign prostatic hyperplasia: a review. Can. J. Urol. 2015;22(Suppl 1):7–17. [PubMed] [Google Scholar]
- 72.Fazio L., Brock G. Erectile dysfunction: management update. CMAJ (Can. Med. Assoc. J.) 2004;170:1429–1437. doi: 10.1503/cmaj.1020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J., Wang Y.B., Ma C.G., Liu T., Li W.R., Gong Y.Q., Xin Z.C. Icarisid II, a PDE5 inhibitor from Epimedium wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue. Andrologia. 2012;44(Suppl 1):87–93. doi: 10.1111/j.1439-0272.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 74.Chen C.Y., Chang Y.H., Bau D.T., Huang H.J., Tsai F.J., Tsai C.H., Chen C.Y. Discovery of potent inhibitors for phosphodiesterase 5 by virtual screening and pharmacophore analysis. Acta Pharmacol. Sin. 2009;30:1186–1194. doi: 10.1038/aps.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Punyawudho B., Puttilerpong C., Wirotsaengthong S., Aramwit P. A randomized, double-blind, placebo-controlled crossover study of Cappra(R) for the treatment of mild or mild to moderate erectile dysfunction in Thai male. Afr J Tradit Complement Altern Med. 2013;10:310–315. [PMC free article] [PubMed] [Google Scholar]
- 76.Nishimatsu H., Kitamura T., Yamada D., Nomiya A., Niimi A., Suzuki M., Fujimura T., Fukuhara H., Nakagawa T., Enomoto Y., Kume H., Igawa Y., Homma Y. Improvement of symptoms of aging in males by a preparation LEOPIN ROYAL containing aged garlic extract and other five of natural medicines - comparison with traditional herbal medicines (Kampo) Aging Male. 2014;17:112–116. doi: 10.3109/13685538.2013.771328. [DOI] [PubMed] [Google Scholar]
- 77.Burbos N., Morris E.P. BMJ Clin Evid; 2011. Menopausal Symptoms; p. 2011. [PMC free article] [PubMed] [Google Scholar]
- 78.Santoro N., Epperson C.N., Mathews S.B. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44:497–515. doi: 10.1016/j.ecl.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaunitz A.M., Manson J.E. Management of menopausal symptoms. Obstet. Gynecol. 2015;126:859–876. doi: 10.1097/AOG.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan F.F., Liu Y., Liu Y.F., Zhao Y.X. Herba Epimedii water extract elevates estrogen level and improves lipid metabolism in postmenopausal women. Phytother Res. 2008;22:1224–1228. doi: 10.1002/ptr.2451. [DOI] [PubMed] [Google Scholar]
- 81.Wang H.Z., Zhang Q., Chen W., Yang X.G., Chen F., Zhuang W. Research progress on sex-hormone-like effects of epimedium. International Journal of Traditional Chinese Medicine. 2022;44:465–468. [Google Scholar]
- 82.Zhang S., Lv B., Huang X., Yang K., Geng Q. Effect of icariin complex on premature ejaculation. Chinese Journal of Andrology. 2010;24:58–59. [Google Scholar]
- 83.Tan X., Weng W. vol. 23. Hunan Yi Ke Da Xue Xue Bao; 1998. pp. 450–452. ([Efficacy of Epimedium Compound Pills in the Treatment of the Aged Patients with Kidney Deficiency Syndrome of Ischemic Cardio-Cerebral Vascular Diseases]). [PubMed] [Google Scholar]
- 84.Ying Z., Zheng G. Liver damage caused by Zhuangguguanjie Wan and cost analysis. Adverse Drug Reactions Journal. 2000 [Google Scholar]
- 85.Beer C., Blacker D., Bynevelt M., Hankey G.J., Puddey I.B. Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J. Neuroinflammation. 2010;7:71. doi: 10.1186/1742-2094-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwang Y.H., Yang H.J., Yim N.H., Ma J.Y. Genetic toxicity of epimedium koreanum Nakai. J. Ethnopharmacol. 2017;198:87–90. doi: 10.1016/j.jep.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 87.Arundine M., Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell. Mol. Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]