Abstract
Aspartame is widely used artificial sweetener. However, chronic exposure to aspartame led to spatial memory impairment and elevated oxidative stress in the brain. Extract of turmeric rhizome (Curcuma longa) (TUR) and extract of bitter melon (Momordica charantia) (BM) is known to have antioxidant activity. The present study was aimed to examine the neuroprotective potential of TUR and BM extracts, either as single or as combination, against the effects of aspartame in the brain. Here, Sprague-Dawley rats fed with aspartame (40 mg/kg BW) for 28 days were compared with rats fed with extract and aspartame. To assess neuroprotective potential, rats were given extract 7 days before and during aspartame treatment. Spatial memory was assessed with Morris water maze test followed with H&E staining of hippocampal region. Brain lipid peroxidation and enzymatic activity of malondialdehyde (MDA), glutathione peroxidase (GPx), and Acetylcholinesterase (AChE) were measured to probe status of oxidative stress in the brain. Aspartame-treated rats demonstrated spatial memory impairment and reduced number of hippocampal cells and elevated levels of MDA, downregulated activity of GPx and elevated activity of AChE. In contrast, animals received both aspartame and extract demonstrated better spatial memory function, higher number of hippocampal areas, increased GPX activity, reduced MDA levels, and decreased AChE activity were observed in the brain of extract-treated rats. Taken together, our results suggest that extract of TUR rhizome and BM fruit exhibit antioxidant activity which may contribute to the neuroprotective effects against aspartame-induced memory impairment in rats.
Keywords: Antioxidant, Curcuma longa, Momordica charantia, Morris water maze, Neuroprotective, Oxidative stress
1. Introduction
Aspartame (ASP) is an artificial sweetener added to various products including beverages, multivitamins, cereal, pudding, and pharmaceuticals worldwide [1,2]. Upon ingestion, ASP is immediately absorbed from the intestinal lumen and metabolized to its metabolite, such as: phenylalanine, aspartate, and methanol [3]. Elevated aspartic acid and phenylalanine in the brain enhance the inhibition of synthesis and release of neurotransmitters that regulate neurophysiological activity including dopamine, norepinephrine, and serotonin, which results in brain damage and cognitive impairment [4]. Indeed, there are numerous reports on the toxic effects of ASP has been reported, such as: induction of neurotoxicity in the brain and sciatic nerve structure due to oxidative stress [2,3,5]. Previous studies also revealed the damaging effects of aspartame at high concentrations on the cerebral and cerebellar cortex, as well as on acetylcholinesterase (AChE) activity in an animal model, which led to impaired spatial memory, learning, and behavior [2,[5], [6], [7]]. Taken together, these studies show that the sweetener might affect brain neurotransmitters and receptors and that these effects may become more prominent following long-term consumption.
Traditional medicine has shown promise in the development of new drugs as it offers unique biological active compounds that improve patient quality of life [8,9]. According to Randino et al. (2016), the curcumin from turmeric rhizome (TUR, Curcuma longa) reduces oxidative damage and age-linked cognitive deficits [10]. In addition, curcumin has been shown to diminish oxidative damage and inhibit β-amyloid aggregation in Alzheimer's disease [11,12].
The bitter melon (BM) fruit, Momordica charantia, has neuroprotective activity through inhibition of lipid peroxide formation and AChE activity [13,14]. The active compound in BM fruit consists of heteropolysaccharides (galactose, glucose, arabinose, rhamnose, and mannose), proteins and peptides (momordin, momorkarins, MAP30, and lectins), terpenes, saponins, flavonoids (quercetin, isorhamnetin), phenolic compounds, as well as other components including amino acids, sterols, essential oils, fatty acids, charantin, vitamin C, nicotinic acid, P alanine, and gamma-aminobutyric acid [15,16]. Charantin [17] and flavonoids [18] were particularly shown to exert neuroprotective activity.
To date, multi-herbal remedies application has been discussed and combining extracts with similar or synergistic activity has attracted interest as an alternative strategy to improve efficacy [19]. However, the combination of TUR rhizome and BM fruit for protecting memory impairment has not been examined. Therefore, we aim to determine the neuroprotective effects of single and combination of extracts as an alternative treatment for spatial memory impairment induced by long term aspartame consumption. In details, the current study was conducted by treating the ASP-induced rats with TUR rhizome and BM fruit before and during ASP induction to investigate the protective effect.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley (SD) albino rats (150–250 g) were obtained from the National Agency of Drug and Food Control, Republic of Indonesia (BPOM RI). All animals were housed and acclimatized for one week in the condition of 12:12 h light-dark cycle, temperature of 24°C-26 °C, and a humidity of 60%–65 %. The rats were feed ad libitum throughout the study. The Ethics Committee of the Faculty of Medicine, Universitas Indonesia has approved this animal experiments with approval number KET-1411/UN2.F1/ETIK/PPM.00.02/2020. The animal handling and procedures were conducted in compliance with the Animal Act of 1986 (scientific procedures).
2.2. Extraction of the turmeric rhizome and bitter melon fruit
The dried powder of TUR rhizome and BM fruit were obtained from the Indonesian Spice and Medicinal Crops Research Institute (Balitro, Bogor, Indonesia) and were taxonomically verified at the Department of Biology, Mathematics and Life Science, Universitas Indonesia (Number 486/UN2.F3.11/PDP.02.00/2020). A 500 g of each dried powder (TUR and BM) was macerated with 96 % ethanol (Merck, Germany) for 24 h and filtered. The filtrate was then concentrated with a rotary vacuum evaporator (Heidolph, Schwabach, Germany) at 40 °C to acquire the solvent-free viscous extracts of TUR rhizome and BM fruit. The extracts were then weighed, and the percentage yield was then calculated.
2.3. Determination of total curcumin content in the ethanolic extract of turmeric rhizome
The total curcumin content in the ethanolic extract of TUR rhizome were determined with thin-layer chromatography scanner system (CAMAG, Muttenz, Switzerland). Briefly, TUR rhizome extract (5 mg/ml) and a curcumin standard solution (0.2–1.4 mg/ml) were spotted onto a silica GF254 plate and eluted with chloroform and methanol (9:1). The bands were then visualized under UV light at 426 nm and the Rf values were calculated. The results were expressed as total curcumin content (mg of total curcumin/100 mg of extract).
2.4. Determination of total flavonoid content in the ethanolic extract of bitter melon fruit
The total flavonoid content of the ethanolic extract of BM fruit were determined using the flavonoid quercetin as the standard (40–120 ppm). The assay was adapted from Tan et al. (2014) with modification [20]. Serial concentrations of standard solutions (1 ml) were combined with 1 ml of 10 % AlCl3, 1 ml of 5 % acetic acid, and 96 % ethanol in a final volume of 10 ml. Meanwhile, 1 ml of the ethanolic extract (1000 ppm) was combined with 1 ml of 10 % AlCl3 and 8 ml of 5 % acetic acid. The absorbance at 510 nm were then measured with Spectrophotometric Jasco V530 UV–Vis instrument (Japan). The total flavonoid content was expressed as quercetin equivalents (mg of quercetin equivalents/100 mg of extract).
2.5. Experimental design
A total of 48 rats were randomly divided into eight groups consisting of six rats per group. Here we used aspartame to impair memory and citicoline as neuroprotective agent. In details, the ethanolic extracts of TUR, BM fruit, and citicoline suspended in 0.5 % carboxymethyl-cellulose natrium (CMC-Na), were administered once daily over the 35 days of the experiment, whereas aspartame was administered daily from day 8 following extract treatment and continued for another 28 days [21]. Fig. 1A shows the design of the animal study. For the dose of the extract, we refer to the previous studies [22,23]. On day 36, spatial memory was analyzed using the MWM test for six days prior to sacrification and dissection of the brains. Cerebral halves of the globe were obtained. Homogenates were then prepared from the cerebral tissue using a Potter–Elvehjem tissue grinder at 4 °C for further analysis.
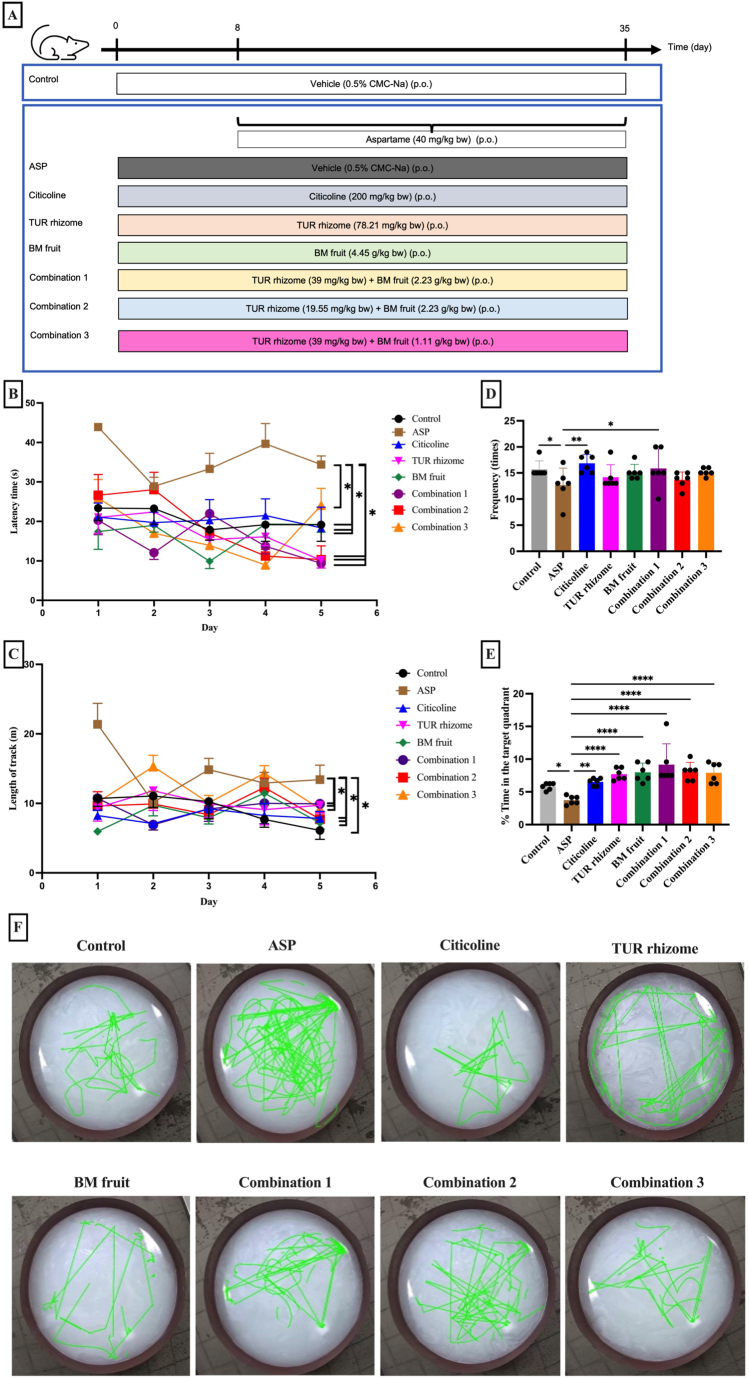
Fig. 1.
The neuroprotective effects of TUR rhizome and BM fruit extracts in combination on spatial memory impairment in the MWM test of rats induced with aspartame. A: Design of the animal experiments B: Latency time profile, and C: Length of track, for the acquisition trial. D: Frequency of crossing the target quadrant, E: Percentage of time in the target quadrant, for the probe trial. F: The swimming trajectory. All data are expressed as means ± SEM, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
2.6. Spatial memory test
We used the MWM apparatus to assess spatial memory by adopting a method from Vorhees & Williams (2006) [24]. The MWM pool with a diameter of 145 cm and 45 cm in height was divided into four quadrants: North, East, South, and West. The camera was placed above the maze. The acquisition trial (self-salvage test) using a 15 cm platform in diameter were used to measure latency time for each animal to find the hidden platform and the track length. These trials were performed for five days at 24 h after the last treatment with aspartame. Briefly, the rats were released into the water randomly facing the maze wall in one quadrant. When the rats were released, a timer was started and stopped once they found the platform. The rats were trained for four consecutive days with four trials in one day to find the platform within the time limit. On day 5, the animals’ spatial memory was assessed through measurements of latency time and the length of track. The latency time and the length of track were denoted as the time required and were denoted as the distance travelled to reach the platform, respectively. The probe trial was intended to determine the spatial memory of the rats. After the acquisition trials, a probe trial was conducted on day six with the same procedure, but without the platform. The rats were placed at a random quadrant in the maze facing the wall. We recorded the time of rats remaining in the quadrant where the previous platform was placed, which was expressed as a percentage of the time spent in the target quadrant, as well as the frequency of crossing the target quadrant. Each animal was tested four times.
2.6.1. Measurement of MDA levels in the left cerebral homogenate
Malondialdehyde (MDA) level were measured according to the method described Nagababu et al. (2010) [25] with slight modification 100 mg of cerebral tissue homogenate was dissolved in 300 μl aquabidest and mixed with 200 μl of 20 % trichloroacetic acid (TCA). The mixture was centrifuged for 10 min at 5000 rpm. The supernatant was combined with 400 μl of 0.6 % thiobarbituric acid and incubated at 96°C-100 °C for 10 min. The MDA levels were calculated as nmol/mg tissue by measuring the absorbance at 532 nm using a spectrophotometer.
2.6.2. Measurement of GPx activity in the left cerebral homogenate
GPx activity was measured with commercial assay kit (RANSEL, RS 505 RANDOX) in accordance with manufacturer instruction. Approximately 100 mg of cerebral tissue homogenate was dissolved in 0.2 ml cold assay buffer and centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was collected to run the assay. The specific GPx enzymatic activity was expressed as U/mg tissue.
2.6.3. Measurement of AChE activity in the left cerebral homogenate
The AChE activity assay was performed with DNTB reagent (5,5-dithiobis(2-nitrobenzoate)) [26]. Cerebral tissue homogenate was added to 2.7 ml of phosphate buffer (pH 8) and then mixed with 0.1 ml of DTNB. Next, 0.1 ml of acetylcholine iodide (pH 8) was added to the mixture and the absorbance was measured at a wavelength of 412 nm. The results were calculated using the following equation:
R = The numbers of moles substrate hydrolyzed per minute, per gram tissue.
ΔΑ = Absorbance per minute.
Co = Tissue concentration (mg/ml).
2.6.4. Histological examination of the CA1-CA3 hippocampal regions
The cerebral tissue was fixed with 10 % formalin for 24 h, washed in graduated alcohol (from 70 %, 80 %,95 %,100 % each for 24 h). Then, washed with xylene twice, the first was for 24 h and the second was for 4–5 h. Tissue was then embedded in liquid paraffin and was cut for 4–5 μm. For Hematoxylin and Eosin staining, tissues were deparaffinize with xylol twice for 2 min each and ethanol with a graded concentration of 100 %, 95 %, 90 %, 80 %, and 70 % and rehydrated with H2O. Sectioned tissues were then stained with Harris Hematoxylin for 15 min and rinsed with water for 5 min, and was followed with eosin staining for 2 min. Finally, sectioned tissue was dehydrated with graduated Ethanol (70 %, 80 %, 95 %, 100 %) and xylene. After dehydrated with alcohol, samples were incubated with xylol twice. The normal cells and pyknosis cells in the CA1–CA3 hippocampal areas were then counted with an OptiLab Advance V2 microscope camera.
2.7. Statistical analysis
GraphPad Prism version 9 (GraphPad, San Diego, CA) is used to perform statistical analysis with a one-way and two-way analysis of variance followed with Fischer's LSD post-hoc test to compare significant differences between groups. The data are expressed as the mean ± SEM and p values of less than 0.05 were considered as statistically significant.
3. Results
Combination of turmeric extract and bitter melon fruit extracts exerts better neuroprotective effect than single extract treatment on aspartame-induced spatial memory impairment.
To calculate the dose of ethanolic extracts for the animal study, we firstly determine the total curcumin content in turmeric rhizome extract and the total flavonoid content in BM fruit extract. Using TLC, we measured the total curcumin content as 25.57 ± 14.1433 mg of total curcumin/100 mg of extract with final yield of 13.04 %. Using spectrophotometry, we measured total flavonoid content in BM fruit extract as 4.490 ± 0.1861 mg of quercetin equivalents/100 mg of extract with final yield of 12 %.
Next, we assessed rat spatial memory by performing MWM test for five days. Here, to impair rat's spatial memory, we treated rats with aspartame for 28 days [21]. To induce neuroprotective effect, we treated rats with citicoline [27] for a week earlier before and during the aspartame induction. Finally, ethanolic extracts of TUR or BM (single or in combination) were then given for a week earlier before and during aspartame intake (Fig. 1A). Fig. 1B and C shows the data for latency time and length of track for the acquisition trial, whereas the percentage of time in the target quadrant and the frequency of crossing the target quadrant for the probe trial are shown in Fig. 1D and E. In addition, the swimming trajectory of all groups is presented in Fig. 1F.
We observed that rat treated with aspartame (ASP) showed increased latency time and length of the track during the acquisition trial, suggesting that the rat's spatial memory was impaired by aspartame induction. In contrast, the latency time and length of track were decreased in animals that received the citicoline, single extract of TUR rhizome, single extract of BM fruits, or the combination extracts (Fig. 1B and C).
Aspartame induction significantly decreased the percentage of time spent in the quadrant and the frequency of crossing the target quadrant compared with the control group. In contrast, all treated groups exhibited an improvement when compared to the ASP group. A significant elevation in the frequency of crossing the target quadrant was evident in the group of citicoline and the group of combination 1. Moreover, all treated groups significantly enhanced the time spent in the target quadrant when compared to the ASP group. Notably, combination 1 group (TUR rhizome (39 mg/kg BW) and BM fruit (2.23 g/kg BW)) shows better tendency effect in improving spatial memory, which significantly enhanced the frequency of crossing the target quadrant when compared to ASP group (Fig. 1D). These results indicate that combination of TUR rhizome and BM fruit might restore learning and memory dysfunction, and thereby, protect the spatial memory from impairment (Fig. 1D–E).
Treatment with turmeric extract or bitter melon fruit extracts or its combination decreased number of degenerative cells in hippocampus of aspartame-inducted rats without affecting the brain weight.
Brain weight is presented in (Supplementary Fig. 1) as the ratio of wet brain weight to body weight. The ratio value was calculated to investigate the effect on the morphology of the brain. Here, we observed that all treatment groups led to no significant differences in the brain weight, suggesting that our treatment did not affect the brain weight.
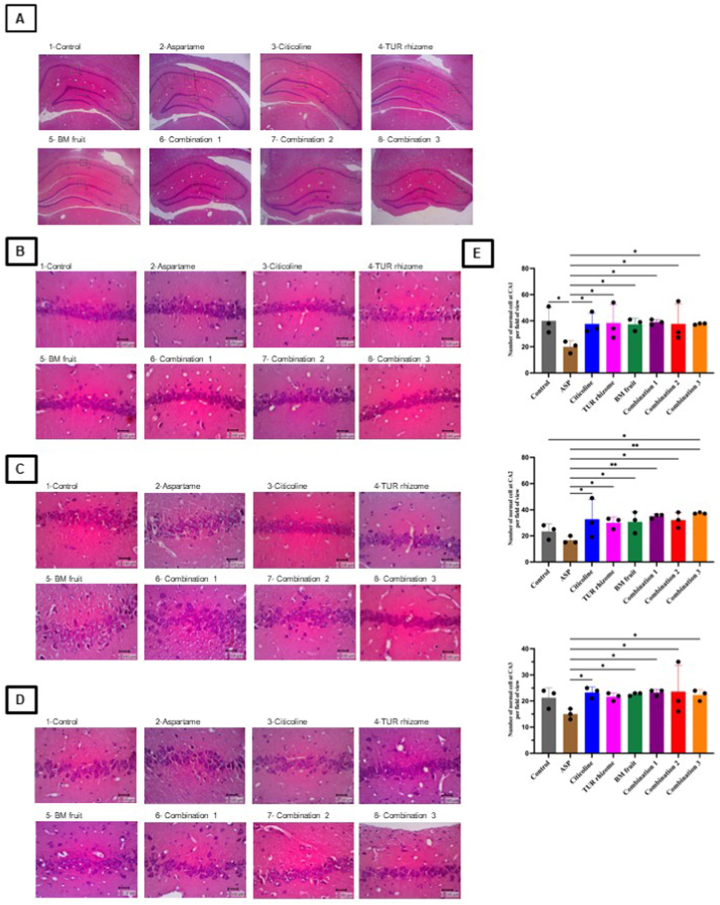
To elucidate how treatment groups exert neuroprotective effect on aspartame-inducted spatial memory impairment, we performed histology analysis in the CA1, CA2, and CA3 hippocampus with the H&E staining method (Fig. 2A–E). We observed cells nuclei appeared as either non-pyknotic/normal or undergoing pyknosis (chromatin condensation), suggesting early apoptosis process on pyknotic cells [28,29]. The total number of cells in the observed area is shown in Fig. 2E. In details, oral administration of citicoline (200 mg/kg BW) significantly increased the total normal cell number (cells that do not show nucleus pyknosis based on the H&E staining) in the areas of CA1 (1.9 fold), CA2 (2 fold) and CA3 (1.5 fold) compared with those in the ASP group. Higher normal cells number also evident in the rats treated with TUR rhizome (CA1:1.9 fold; CA2: 1.8 fold; CA3: 1.4 fold) or treated with BM fruit extracts (CA1:1.9 fold; CA2: 1.8 fold; CA3: 1.5 fold) when compared with the ASP group. Finally, combination treatment, particularly for the Combination 1 group also led to higher number of normal cells when compared with the ASP group (CA1:2 fold; CA2: 2.1 fold; CA3: 1.6 fold). Taken together, our data suggest that the treatment of the TUR rhizome and the BM fruit extracts led to decreased number of degenerative cells in the CA1–CA3 hippocampal areas with the combination of both extracts results in a better effect, especially in the C2 area of aspartame-induced rat's brain.
Fig. 2.
The effects of TUR rhizome and BM fruit extracts in combination on the degenerative cells in hippocampus. A: Hippocampal area in with magnification (×4) observed in all groups, B: H&E-stained sections of all groups in the CA1 with magnification (×40), C: H&E-stained sections of all groups in the CA2 with magnification (×40), D: H&E-stained sections of all groups in the CA3 with magnification (×40), E: Histogram of the number of normal cells in the CA1–CA3 areas. Data are expressed as means ± SEM, n = 6 (brain to body weight ratio), n = 3 (histology analysis). *p < 0.05, **p < 0.01.
Treatment with turmeric extract or bitter melon fruit extracts or its combination led to increased anti-oxidant markers and reduced AChE activity in the brain.
It is suggested chronic exposure of aspartame led to oxidative stress damage in the brain [5,7]. Since our results suggested treatment with extracts exert the neuroprotective potential, we hypothesize our treatment might increase antioxidant level in the brain. Thus, we next measured antioxidant markers, MDA levels and GPx activities, in the brain.
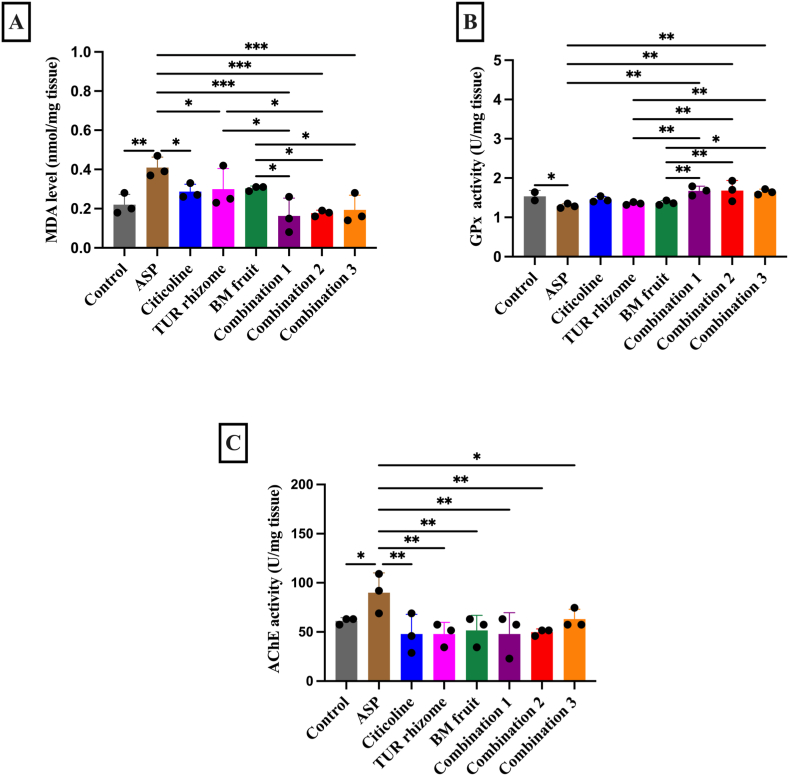
MDA is the final product formed during the cellular lipid peroxidation, which is released into extracellular space and finally into the blood; thus, MDA has been used as an effective oxidative stress marker [30]. Here, we reported that in all treated groups, except BM fruit, had significantly decreased MDA levels when compared to the ASP group. Notably, combination treatment of TUR rhizome and BM fruit extracts resulted in a greater reduction when compared to the single extract group of either TUR rhizome or BM fruit, except for combination 3, which did not significantly differ from TUR rhizome (Fig. 3A).
Fig. 3.
The effects of TUR rhizome and BM fruit extract combination on the oxidative stress and acetylcholinesterase (AChE) biochemical parameters in rat brain. A: Brain MDA levels, B: Brain GPx activity, C: Brain AChE activity. All data are expressed as means ± SEM, n = 3. *p < 0.05, **p < 0.01, ***p < 0.001.
Glutathione peroxidase-1 (GPx-1) is an intracellular antioxidant enzyme that enzymatically reduces hydrogen peroxide to water to limit its harmful effect [31]. Here, we reported that combination treatment of TUR rhizome and BM fruit led to significant elevation of GPx activity when compared to the ASP group (P < 0.05). Single treatment of either extract and citicoline treatment tended to increase, but did no significant difference observed when comparing the mentioned treatments with the ASP group (Fig. 3B). Taken together, our results suggest that extracts treatment, especially, the combination of TUR rhizome and BM fruit, resisted effect of aspartame induction by elevating anti-oxidative stress markers.
AChE is an enzyme catalyzes the reaction of acethyl and choline from acetylcholine. It is involved in learning and memory and widely used as an indicator to detect the neurotoxic effect [32]. Thus, in addition to measuring antioxidant marker, we also measured AChE in rats’ brain. Here, we reported significant elevation in AChE activity in the ASP group versus the control group, implying that consumption of ASP led to neurotoxic effect in the brain. However, all treatments with TUR rhizome and BM fruit extracts, either alone or in combination, significantly reduced AChE activity when compared to the ASP group, implying that our treatment led to neuroprotective effect in the rats brain (Fig. 3C).
4. Discussion
The present study examined the effect of TUR extract and BM fruits on rat exposed to the aspartame in spatial memory function, number of cells in hippocampal cells, and oxidative stress enzyme. In details, results of this study reveal that the administration of aspartame for 28 days to rats led to impaired spatial memory function, reduced number of non-pyknotic cells in the CA1–CA3 hippocampal regions, and alteration of the levels of MDA, GPx, AChE in the brain. In contrast, animals received both aspartame and extract demonstrated amelioration of aspartame effect in tested parameters above.
The impact of aspartame on memory has been examined in humans and experimental animals. A previous study on human subjects reported the increased aspartame consumption led to weaker spatial orientation [33]. Similarly, Collison et al. [34]demonstrated spatial cognition impairment upon long-term exposure to aspartame. In agreement with previous study, administration of aspartame for 28 days led to impaired spatial memory as tested with MWM test. Since the hippocampus is associated with spatial memory function [35], we next checked the number of cells in the hippocampus and noted the reduction of non-pyknotic cells number in the CA1–CA3 hippocampal regions of the aspartame-treated rats. Although we could not confirm the identity of the cells due to lack of staining, previous study has suggested that aspartame treatment led to reduced neuronal cells counts and reactive gliosis in hippocampus [36]. In addition, the pyknosis nucleus is an early sign of apoptosis [29], suggesting that aspartame treatment led to lower number of living cells in the hippocampus. Since Ashok et al. [37] reported that aspartame damage to the CA area is due to elevated levels of free radicals which led to oxidative stress, we next examined the brain oxidative level through brain lipid peroxidation (MDA) [36] and neurotoxin level through measurement of AChE enzymatic activity. Finally, we measured brain GPx activity as a representative for anti-oxidant enzymatic mechanisms, which might compensate for ROS production. As expected, the aspartame-treated brain led to higher level of MDA and AChE and lower level of GPx, similar to previous study. In addition, we used citicoline as control for antioxidant properties [38] against aspartame's harmful effect. In our study, rats treated with citicoline shoed alleviation from aspartame's effect. Taken together, we and other have demonstrated that aspartame consumption might impair memory function through free radical damage in hippocampus. Furthermore, there are numerous reports on damage of aspartame on other body organs such as in kidney, liver and lung which suggesting the needs to create awareness on usage of artificial sweetener in daily life [39,40].
Since aspartame is harmful to the body there is a necessity to establish alternative effort to alleviate aspartame damage against our body. Here, we aim to characterized the effect of TUR and BM extract, either as single or combination against the damage caused by aspartame consumption. Based on the MWM test, both extracts reduced the time that rats required to reach the platform and the length of the track, thus spending more time in the target quadrant, and enhancing the frequency in the target area (Fig. 1). In the hippocampus, treatment with TUR rhizome and BM fruit led to the higher number of normal cells (cells that are not undergo nucleus pyknosis or apoptosis) after aspartame-induction (Fig. 2). Finally, we noted reduction in oxidative stress as evidenced by a decrease in brain MDA levels and enhanced GPx activity, and decreased AChE activity in the brain of extract-treated rats (Fig. 3E). Here, our data echoed previous findings which demonstrated that ethanolic extract of Curcuma longa prevents oxidative stress by reducing brain MDA levels and increasing anti-oxidant enzymes (SOD, CAT, and GPx) activities against trimethyltin-induced oxidative stress in rats [41,42]. In addition, Pratibha et al. [43] demonstrated that BM fruit normalized GPx activity elevation by 40 % in HFD-fed mice exhibiting oxidative stress. Taken together, we showed here that ethanolic extracts of TUR rhizome and BM fruit could be used to ameliorate the spatial memory impairment induced with aspartame, at least in rats.
Here, we confirmed that ethanolic extracts of TUR rhizome contain curcumin at 25.57 ± 14.1433 mg of total curcumin/100 mg of extract whereas the BM fruit extract were confirmed to contain flavonoid, quercetin. Abdalla et al. (2014) [44] demonstrated that quercetin inhibits AChE activity. The inhibition of AChE by quercetin results from the interaction between the OH groups that forms hydrogen bonds with amino acid residues at the active site of the enzyme, which increases acetylcholine levels in the synaptic cleft. Since we noted downregulated AChE activity in extract treated rats, the administration of TUR rhizome and BM fruit may rescue the cholinergic functions through above mechanism. Overall, our results suggest that TUR rhizome and BM fruit through their curcumin and quercetin, respectively, possess antioxidant activity which led to increased GPx activity and reduced MDA levels, which eventually leads to oxidative stress inhibition. This may underlie the mechanisms involved in improving spatial memory impairment in rats exposed to aspartame in our experimental design.
Herbal medicine combinations are widely used, such as in traditional Chinese medicine, by utilizing synergistic, additive, or antagonist interactions with each active compound or formulation [45]. Here, we observed a tendency of combining both extracts treatment led to better effect when compared to a single extract treatment of TUR rhizome (78.21 mg/kg/BW) or BM fruit (4.45 g/kg/BW). In particular, the combination containing TUR rhizome (39 mg/kg/BW) and BM fruit (2.23 g/kg/BW) showed statistical significant in MWM, the most elevated antioxidant activity, and inhibited MDA level and AChE activity when compared with the other groups. The combination of both extracts also resulted in a significant increase in GPx activity, whereas single treatment did not show a statistically significant difference. The use of the extract combination may also be beneficial for increasing the permeability and bioavailability of compounds, such as curcumin. In a previous study by Lund & Pantuso (2014) [46], the combination of curcumin and quercetin in vitro resulted in an increase of 147 % permeability in the intestine, and when combined with quercetin and resveratrol, curcumin increased permeability by 188 %. Thus, the combination of curcumin and quercetin in both extracts may provide synergistic effects in our study.
There are several limitations in our studies that should be addressed in the future studies, such as the comprehensive mechanistic study on TUR rhizome and BM fruit extract, additional assays to evaluate the chemical constituents in single and combination of both extracts, immunostaining to confirm neural cells marker (neuronal marker (NeuN, Tuj1), Nissl staining, glia marker (GFAP, S100b)) as well as apoptotic marker (TUNNEL staining or Annexin V, etc.) in the hippocampus, and limited investigation on other antioxidant enzyme (e.g. CAT, GSH, SOD). In addition, further study should also be conducted to evaluate the possible interactions between both extracts.
5. Conclusion
Here, we demonstrated that the treatment of ethanolic extract of TUR rhizome and BM fruit suppresses spatial memory impairment, led to fewer degenerative cells in the CA1-CA3 hippocampal areas, and inhibition of oxidative stress. In addition, we observed a tendency of combining extract might be better than single treatment, especially for TUR rhizome (39 mg/kg/BW) and BM fruit (2.23 g/kg/BW). This may result from the potent antioxidant activities exhibited by both extracts, which contain curcumin and flavonoids, by reducing MDA levels and increasing GPx activity in the brain. However, since comprehensive mechanism studies still lacking, further studies could explore more on the mechanism of the TUR rhizome and BM fruit extract.
Funding
The funding of this study was supported by the Ministry of Research and Technology of the Republic of Indonesia [PDUPT grant number NKB-060/UN2.RST/HKP.05.00/2021].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank the Faculty of Pharmacy and the Directorate of Research and Development, Universitas Indonesia for supporting this work, and Enago (www.enago.com) for the English language review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21693.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Magnuson B.A., Burdock G.A., Doull J., Kroes R.M., Marsh G.M., Pariza M.W., Spencer P.S., Waddell W.J., Walker R., Williams G.M. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- 2.Okasha E.F. Effect of long term-administration of aspartame on the ultrastructure of sciatic nerve. J Microsc Ultrastruct. 2016;4:175–183. doi: 10.1016/j.jmau.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rycerz Karol J.E., Jaworska-Adamu Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. 2013;51:10–17. doi: 10.5114/fn.2013.34191. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary A.K., Lee Y.Y. Neurophysiological symptoms and aspartame: what is the connection? Nutr. Neurosci. 2018;21:306–316. doi: 10.1080/1028415X.2017.1288340. [DOI] [PubMed] [Google Scholar]
- 5.Iyaswamy A., Kammella A.K., Thavasimuthu C., Wankupar W., Dapkupar W., Shanmugam S., Rajan R., Rathinasamy S. Oxidative stress evoked damages leading to attenuated memory and inhibition of NMDAR–CaMKII–ERK/CREB signalling on consumption of aspartame in rat model. J. Food Drug Anal. 2018;26:903–916. doi: 10.1016/j.jfda.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collison K.S., Makhoul N.J., Zaidi M.Z., Saleh S.M., Andres B., Inglis A., Al-Rabiah R., Al-Mohanna F.A. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onaolapo A.Y., Onaolapo O.J., Nwoha P.U. Alterations in behaviour, cerebral cortical morphology and cerebral oxidative stress markers following aspartame ingestion. J. Chem. Neuroanat. 2016;78:42–56. doi: 10.1016/j.jchemneu.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Pandit S., Kim H.-J., Kim J.-E., Jeon J.-G. Separation of an effective fraction from turmeric against Streptococcus mutans biofilms by the comparison of curcuminoid content and anti-acidogenic activity. Food Chem. 2011;126:1565–1570. doi: 10.1016/j.foodchem.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randino R., Grimaldi M., Persico M., de Santis A., Cini E., Cabri W., Riva A., D'Errico G., Fattorusso C., D'Ursi A.M., Rodriquez M. Investigating the neuroprotective effects of turmeric extract: structural interactions of β-amyloid peptide with single curcuminoids. Sci. Rep. 2016;6 doi: 10.1038/srep38846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005:280. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 12.dong Fan C., Li Y., ting Fu X., jian Wu Q., jun Hou Y., feng Yang M., yi Sun J., yan Fu X., cheng Zheng Z., liang Sun B. Reversal of beta-amyloid-induced neurotoxicity in PC12 cells by curcumin, the important role of ROS-mediated signaling and ERK pathway. Cell. Mol. Neurobiol. 2017;37 doi: 10.1007/s10571-016-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathakota R. Neuroprotective effects of momordica charantia on scopolamine induced ALZHEIMER’S disease. World J. Pharm. Pharmaceut. Sci. 2017:2141–2155. [Google Scholar]
- 14.Huang H.-J., Chen S.-L., Chang Y.-T., Chyuan J.-H., Hsieh-Li H.M. Administration of momordica charantia enhances the neuroprotection and reduces the side effects of LiCl in the treatment of Alzheimer's disease. Nutrients. 2018;10 doi: 10.3390/nu10121888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik R.A., Saad M.M., Tiwari S. Effect of extraction time and solvent power on phytochemical screening and antioxidant activity of momordica charantia l. Fruit extracts. Asian J. Chem. 2019;31:647–650. doi: 10.14233/ajchem.2019.21715. [DOI] [Google Scholar]
- 16.Bortolotti M., Mercatelli D., Polito L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00486. https://www.frontiersin.org/articles/10.3389/fphar.2019.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamilanban T. In vitro neuroprotective effect of charantin from Momordica charantia against neurotoxin and endoplasmic reticulum stress-induced cell death in SH-SY5Y cells. Int. J. Green Pharm. 2018;12 [Google Scholar]
- 18.Kale A., Pişkin Ö., Baş Y., Aydın B.G., Can M., Elmas Ö., Büyükuysal Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018;59:404–410. doi: 10.1093/jrr/rry032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirawanchai N., Kengkoom K., Isarangkul D., Burana-osot J., Kanjanapruthipong T., Chantip S., Phattanawasin P., Sotanaphun U., Ampawong S. A combination extract of kaffir lime, galangal, and lemongrass maintains blood lipid profiles, hepatocytes, and liver mitochondria in rats with nonalcoholic steatohepatitis. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109843. [DOI] [PubMed] [Google Scholar]
- 20.Tan S., Parks S., Stathopoulos C., Roach P. Extraction of flavonoids from bitter melon. Food Nutr. Sci. 2014;5:458–465. doi: 10.4236/fns.2014.55054. [DOI] [Google Scholar]
- 21.Vicko S., Fadlina, Saputri F., Mun’im A. Jurnal Aisyah Jurnal Ilmu Kesehatan; 2021. Memory Loss Induced by Aspartame in Albino Rats: Study on Neurobehavioral Changes; p. 364. [DOI] [Google Scholar]
- 22.Xu Y., Ku B., Tie L., Yao H., Jiang W., Ma X., Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Chuanfeng L., Hong T., Yang Z., Zhang Y., Wang L., Dong M., Zhao J., Mu J., Meng Y. 2012. Effect of Quercetin in Mouse Model of Parkinson ’ S Disease, Evidence-Based Complementary and Alternative Medicine; p. 2012. [DOI] [Google Scholar]
- 24.v Vorhees C., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagababu E., Rifkind J.M., Boindala S., Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 2010;610 doi: 10.1007/978-1-60327-029-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Diederich K., Frauenknecht K., Minnerup J., Schneider B.K., Schmidt A., Altach E., Eggert V., Sommer C.J., Schäbitz W.-R. Citicoline enhances neuroregenerative processes after experimental stroke in rats. Stroke. 2012;43:1931–1940. doi: 10.1161/STROKEAHA.112.654806. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler U., Groscurth P. vol. 19. News in Physiological Sciences; 2004. (Morphological Features of Cell Death). [DOI] [PubMed] [Google Scholar]
- 29.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35 doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metabol. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.H., Cha M., Lee B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21197152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Q., Liao C., Ge F., Ao J., Liu T. Acetylcholine bidirectionally regulates learning and memory. J. Neurorestoratol. 2022;10 doi: 10.1016/j.jnrt.2022.100002. [DOI] [Google Scholar]
- 33.Lindseth G.N., Coolahan S.E., Petros T.V., Lindseth P.D. Neurobehavioral effects of aspartame consumption. Res. Nurs. Health. 2014;37:185–193. doi: 10.1002/nur.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collison K.S., Makhoul N.J., Zaidi M.Z., Saleh S.M., Andres B., Inglis A., Al-Rabiah R., Al-Mohanna F.A. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird C.M., Burgess N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 2008;9 doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 36.Erbaş O., Erdoğan M.A., Khalilnezhad A., Solmaz V., Gürkan F.T., Yiğittürk G., Eroglu H.A., Taskiran D. Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22053. [DOI] [PubMed] [Google Scholar]
- 37.Ashok I., Sheeladevi R., Wankhar D. Long-term effect of aspartame (artificial sweetener) on ATPases, antioxidant status and histopathology in the rat brain. Free Radic. Antioxidants. 2014;3 doi: 10.1016/j.fra.2013.09.003. S42–S49. [DOI] [Google Scholar]
- 38.Zhong Z., Fu X., Li H., Chen J., Wang M., Gao S., Zhang L., Cheng C., Zhang Y., Li P., Zhang S., Qian X., Shu Y., Chai R., Gao X. Citicoline protects auditory hair cells against neomycin-induced damage. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-ghaffar S.Kh, Adly M.A., El-Sayed M.F., Abd-Elsamei W.M. Protective effects of some antioxidants against long-term intake of aspartame toxicity on liver and kidney: biochemical and histopathological approach in rats. The Journal of Basic and Applied Zoology. 2021;82 doi: 10.1186/s41936-021-00244-9. [DOI] [Google Scholar]
- 40.Mousa H., Abdelfadeel K., Hassan M. Green kiwi fruit extract ameliorates aspartame toxicity on the lung of adult male abino rat (histological and immunohistochemical study) Journal of Medical Histology. 2019;3 doi: 10.21608/jmh.2019.15854.1063. [DOI] [Google Scholar]
- 41.Yuliani S., Mustofa, Partadiredja G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr. Neurosci. 2019;22 doi: 10.1080/1028415X.2018.1447267. [DOI] [PubMed] [Google Scholar]
- 42.Madiha S., Batool Z., Tabassum S., Liaquat L., Sadir S., Perveen T., Haider S. Therapeutic effects of Curcuma longa against rotenone-induced gross motor skill deficits in rats. Pakistan J. Zool. 2018;50 doi: 10.17582/journal.pjz/2018.50.4.1245.1256. [DOI] [Google Scholar]
- 43.Nerurkar P.V., Johns L.M., Buesa L.M., Kipyakwai G., Volper E., Sato R., Shah P., Feher D., Williams P.G., Nerurkar V.R. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J. Neuroinflammation. 2011;8 doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdalla F.H., Schmatz R., Cardoso A.M., Carvalho F.B., Baldissarelli J., de Oliveira J.S., Rosa M.M., Gonçalves Nunes M.A., Rubin M.A., da Cruz I.B.M., Barbisan F., Dressler V.L., Pereira L.B., Schetinger M.R.C., Morsch V.M., Gonçalves J.F., Mazzanti C.M. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na+,K+-ATPase activities. Physiol. Behav. 2014;135:152–167. doi: 10.1016/j.physbeh.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X., Seto S.W., Chang D., Kiat H. Synergistic effects of Chinese herbal medicine. A Comprehensive Review of Methodology and Current. 2016;7:1–16. doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund K.C., Pantuso T. Combination effects of quercetin, resveratrol and curcumin on in vitro intestinal absorption. J Restor Med. 2014;3:112–120. doi: 10.14200/jrm.2014.3.0108. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.